This is a preprint.
Endothelial Dysfunction in Breast Cancer Survivors on Aromatase Inhibitors: Changes over Time
- PMID: 37066265
- PMCID: PMC10104271
- DOI: 10.21203/rs.3.rs-2758909/v1
Endothelial Dysfunction in Breast Cancer Survivors on Aromatase Inhibitors: Changes over Time
Update in
-
Endothelial dysfunction in breast cancer survivors on aromatase inhibitors: changes over time.Cardiooncology. 2024 May 1;10(1):27. doi: 10.1186/s40959-024-00227-z. Cardiooncology. 2024. PMID: 38693561 Free PMC article.
Abstract
Background: Aromatase inhibitors (AIs) are recommended as adjuvant treatment for estrogen-receptor positive breast carcinoma in postmenopausal women. Studies demonstrate mixed results as to the impact of AIs on cardiovascular (CV) events and overall survival. With the increasing number of pre- and postmenopausal women on AIs for five to ten years, understanding the long-term impact of AIs on blood vessels and CV risk in cancer survivors is vital.
Methods: A single arm longitudinal study of 14 postmenopausal women with ER+ breast cancer prescribed adjuvant AIs at the University of Minnesota. Subjects with a history of tobacco use, hypertension, or hyperlipidemia were excluded. Participants underwent routine labs, blood pressure assessments, and vascular testing at baseline (prior to starting AIs) and at six months. Vascular assessment was performed using the EndoPAT 2000 and HDI/PulseWave CR-2000 Cardiovascular Pro ling System and pulse contour analysis on two occasions as previously described. Vascular measurements were conducted by one trained vascular technician. Assessments were performed in triplicate, and the mean indices were used for analyses. All subjects were on an AI at the follow-up visit. The protocol was approved by the UMN Institutional Review Board and all participants were provided written informed consent. Baseline and follow-up characteristics were compared using Wilcoxon signed-rank tests. Analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
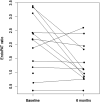
Results: After six months of AI treatment, EndoPAT® ratio declined to a median 1.12 (Q1: 0.85, Q3: 1.86; p=0.045) and median estradiol levels decreased to 2 pg/mL (Q1: 2, Q3: 3; p=0.052). There was no evidence of association between change in EndoPAT® and change in estradiol level (p=0.91). There were no statistically significant changes in small or large arterial elasticity.
Conclusion: Endovascular dysfunction is an early sign for atherosclerosis and vascular impairment. This study suggests that postmenopausal breast cancer survivors on aromatase inhibitor therapy develop endothelial dysfunction as early as six months which is a predictor of adverse CV disease. We hypothesize that long-term use of AIs can lead to persistent endothelial dysfunction. It is unclear if these changes are reversible once AI use is discontinued and further investigation is necessary.
Keywords: aromatase inhibitors; breast cancer; cardiotoxicity; endothelial function.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests
Figures
Similar articles
-
Endothelial dysfunction in breast cancer survivors on aromatase inhibitors: changes over time.Cardiooncology. 2024 May 1;10(1):27. doi: 10.1186/s40959-024-00227-z. Cardiooncology. 2024. PMID: 38693561 Free PMC article.
-
Vascular function in breast cancer survivors on aromatase inhibitors: a pilot study.Breast Cancer Res Treat. 2017 Nov;166(2):541-547. doi: 10.1007/s10549-017-4447-6. Epub 2017 Aug 11. Breast Cancer Res Treat. 2017. PMID: 28801846 Free PMC article.
-
The role of aromatase inhibitors in early breast cancer.Curr Treat Options Oncol. 2003 Apr;4(2):133-40. doi: 10.1007/s11864-003-0014-y. Curr Treat Options Oncol. 2003. PMID: 12594939 Review.
-
Attenuated peripheral endothelial function among women treated with aromatase inhibitors for breast cancer.Coron Artery Dis. 2018 Dec;29(8):687-693. doi: 10.1097/MCA.0000000000000666. Coron Artery Dis. 2018. PMID: 30379695
-
Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer.Cochrane Database Syst Rev. 2020 Jan 29;1(1):CD012988. doi: 10.1002/14651858.CD012988.pub2. Cochrane Database Syst Rev. 2020. PMID: 31994181 Free PMC article.
References
-
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019. - PubMed
-
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. The lancet oncology. 2010;11(12):1135–1141. - PubMed
-
- Meyer MR, Barton M. Estrogens and Coronary Artery Disease: New Clinical Perspectives. Adv Pharmacol. 2016;77:307–360. - PubMed
-
- Khosrow-Khavar F, Filion KB, Bouganim N, Suissa S, Azoulay L. Aromatase Inhibitors and the Risk of Cardiovascular Outcomes in Women With Breast Cancer: A Population-Based Cohort Study. Circulation. 2020;141(7):549–559. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources