This is a preprint.
Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a NO-CCL2-CCR2 pathway
- PMID: 37066295
- PMCID: PMC10103986
- DOI: 10.1101/2023.04.03.535167
Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a NO-CCL2-CCR2 pathway
Update in
-
Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a CCL2-CCR2 pathway and NO dysregulation.Cell Rep. 2024 May 28;43(5):114193. doi: 10.1016/j.celrep.2024.114193. Epub 2024 May 5. Cell Rep. 2024. PMID: 38709635 Free PMC article.
Abstract
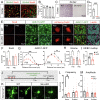
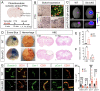
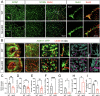
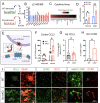
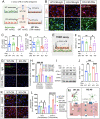
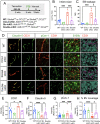
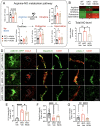
Astrocytes play vital roles in blood-brain barrier (BBB) maintenance, yet how they support BBB integrity under normal or pathological conditions remains poorly defined. Recent evidence suggests pH homeostasis is a new cellular mechanism important for BBB integrity. In the current study, we investigated the function of an astrocyte-specific pH regulator, Slc4a4, in BBB maintenance and repair. We show that astrocytic Slc4a4 is required for normal astrocyte morphological complexity and BBB function. Multi-omics analyses identified increased astrocytic secretion of CCL2 coupled with dysregulated arginine-NO metabolism after Slc4a4 deletion. Using a model of ischemic stroke, we found that loss of Slc4a4 exacerbates BBB disruption and reactive gliosis, which were both rescued by pharmacological or genetic inhibition of the NO-CCL2 pathway in vivo. Together, our study identifies the astrocytic Slc4a4-NO-CCL2 axis as a pivotal mechanism controlling BBB integrity and repair, while providing insights for a novel therapeutic approach against BBB-related CNS disorders.
Keywords: Astrocyte; CCL2; Nitric oxide; Slc4a4; blood-brain barrier; ischemic stroke; pH regulation.
Conflict of interest statement
Conflicts of interests Y.K.R. is the founder of pHLIP Inc., but the company did not fund any part of the work reported here. The authors declare no other competing interests.
Figures


References
-
- Daneman R, Engelhardt B. Brain barriers in health and disease. Neurobiol. Dis. 2017;107:1–3. - PubMed
-
- Gorelick PB. The global burden of stroke: persistent and disabling. [Internet]. Lancet. Neurol. 2019;18(5):417–418. - PubMed
-
- Hankey GJ. Stroke. [Internet]. Lancet (London, England) 2017;389(10069):641–654. - PubMed
-
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10(11):1369–1376. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
