This is a preprint.
Mrs4 loss of function in fungi during adaptation to the cystic fibrosis lung
- PMID: 37066389
- PMCID: PMC10104081
- DOI: 10.1101/2023.04.05.535776
Mrs4 loss of function in fungi during adaptation to the cystic fibrosis lung
Update in
-
Mrs4 loss of function in fungi during adaptation to the cystic fibrosis lung.mBio. 2023 Aug 31;14(4):e0117123. doi: 10.1128/mbio.01171-23. Epub 2023 Jul 11. mBio. 2023. PMID: 37432019 Free PMC article.
Abstract
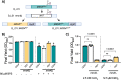
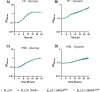
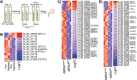
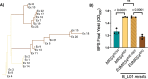
The genetic disease cystic fibrosis (CF) frequently leads to chronic lung infections by bacteria and fungi. We identified three individuals with CF with persistent lung infections dominated by Clavispora ( Candida ) lusitaniae . Whole genome sequencing analysis of multiple isolates from each infection found evidence for selection for mutants in the gene MRS4 in all three distinct lung-associated populations. In each population, we found one or two unfixed, non-synonymous mutations in MRS4 relative to the reference allele found in multiple environmental and clinical isolates including the type strain. Genetic and phenotypic analyses found that all evolved alleles led to loss of function of Mrs4, a mitochondrial iron transporter. RNA Seq analyses found that Mrs4 variants with decreased activity led to increased expression of genes involved in iron acquisition mechanisms in both low iron and replete iron conditions. Furthermore, surface iron reductase activity and intracellular iron was much higher in strains with Mrs4 loss of function variants. Parallel studies found that a subpopulation of a CF-associated Exophiala dermatiditis infection also had a non-synonymous loss of function mutation in MRS4. Together, these data suggest that MRS4 mutations may be beneficial during chronic CF lung infections in diverse fungi perhaps for the purposes of adaptation to an iron restricted environment with chronic infections.
Figures



References
-
- Kim SH, Clark ST, Surendra A, Copeland JK, Wang PW, Ammar R, Collins C, Tullis DE, Nislow C, Hwang DM, Guttman DS, Cowen LE. 2015. Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation. PLoS Pathog 11:e1005308. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
