This is a preprint.
Dermis resident macrophages orchestrate localized ILC2-eosinophil circuitries to maintain their M2-like properties and promote non-healing cutaneous leishmaniasis
- PMID: 37066418
- PMCID: PMC10104262
- DOI: 10.21203/rs.3.rs-2644705/v1
Dermis resident macrophages orchestrate localized ILC2-eosinophil circuitries to maintain their M2-like properties and promote non-healing cutaneous leishmaniasis
Update in
-
Dermis resident macrophages orchestrate localized ILC2 eosinophil circuitries to promote non-healing cutaneous leishmaniasis.Nat Commun. 2023 Nov 29;14(1):7852. doi: 10.1038/s41467-023-43588-2. Nat Commun. 2023. PMID: 38030609 Free PMC article.
Abstract
Tissue-resident macrophages (TRMs) are critical for tissue homeostasis/repair. We previously showed that dermal TRMs produce CCL24 (eotaxin2) which mediates their interaction with IL-4 producing eosinophils, required to maintain their number and M2-like properties in the TH1 environment of the Leishmania major infected skin. Here, we unveil another layer of TRM self-maintenance involving their production of TSLP, an alarmin typically characterized as epithelial cell-derived. Both TSLP signaling and IL-5+ innate lymphoid cell 2 (ILC2s) were shown to maintain the number of dermal TRMs and promote infection. Single cell RNA sequencing identified the dermal TRMs as the sole source of TSLP and CCL24. Development of Ccl24-cre mice permitted specific labeling of dermal TRMs, as well as interstitial TRMs from other organs. Genetic ablation of TSLP from dermal TRMs reduced the number of dermal TRMs, and disease was ameliorated. Thus, by orchestrating localized type 2 circuitries with ILC2s and eosinophils, dermal TRMs are self-maintained as a replicative niche for L. major.
Keywords: CCL24; IL-4; IL-5; ILC2; Leishmania major; TSLP; Tissue-resident macrophages; eosinophil.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




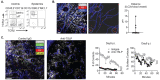


References
-
- Sabatel C. et al. Exposure to Bacterial CpG DNA Protects from Airway Allergic Inflammation by Expanding Regulatory Lung Interstitial Macrophages. Immunity 46, 457–473 (2017). - PubMed
-
- Tamoutounour S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

