This is a preprint.
Probiotic-derived ecto-5'-nucleotidase produces anti-inflammatory adenosine metabolites in Treg-deficient scurfy mice
- PMID: 37066419
- PMCID: PMC10104250
- DOI: 10.21203/rs.3.rs-2781715/v1
Probiotic-derived ecto-5'-nucleotidase produces anti-inflammatory adenosine metabolites in Treg-deficient scurfy mice
Update in
-
Probiotic-Derived Ecto-5'-Nucleotidase Produces Anti-Inflammatory Adenosine Metabolites in Treg-Deficient Scurfy Mice.Probiotics Antimicrob Proteins. 2023 Aug;15(4):1001-1013. doi: 10.1007/s12602-023-10089-z. Epub 2023 May 13. Probiotics Antimicrob Proteins. 2023. PMID: 37178405 Free PMC article.
Abstract
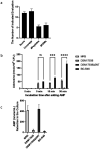
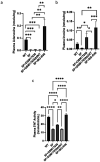
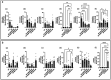
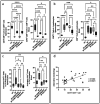
Probiotic Limosilactobacillus reuteri DSM 17938 (DSM 17938) prolonges the survival of Treg-deficient scurfy (SF) mice and reduces multiorgan inflammation by a process requiring adenosine receptor 2A (A 2A ) on T cells. We hypothesized that L. reuteri -derived ecto-5'-nucleotidase (ecto-5'NT) activity acts to generate adenosine, which may be a central mediator for L. reuteri protection in SF mice. We evaluated DSM 17938-5'NT activity and the associated adenosine and inosine levels in plasma, gut and liver of SF mice. We examined orally fed DSM 17938, DSM 17938Δ5NT (with a deleted 5'NT gene), and DSM 32846 (BG-R46) (a naturally selected strain derived from DSM 17938). Results showed that DSM 17938 and BG-R46 produced adenosine while "exhausting" AMP, whereas DSM 17938∆5NT did not generate adenosine in culture. Plasma 5'NT activity was increased by DSM 17938 or BG-R46, but not by DSM 17938Δ5NT in SF mice. BG-R46 increased both adenosine and inosine levels in the cecum of SF mice. DSM 17938 increased adenosine levels, whereas BG-R46 increased inosine levels in the liver. DSM 17938Δ5NT did not significantly change the levels of adenosine or inosine in the GI tract or the liver of SF mice. Although regulatory CD73 + CD8 + T cells were decreased in spleen and blood of SF mice, these regulatory T cells could be increased by orally feeding DSM 17938 or BG-R46, but not DSM 17938Δ5NT. In conclusion, probiotic-5'NT may be a central mediator of DSM 17938 protection against autoimmunity. Optimal 5'NT activity from various probiotic strains could be beneficial in treating Treg-associated immune disorders in humans.
Conflict of interest statement
Stefan Roos has part-time employment by BioGaia AB, Stockholm, Sweden. Jan-Peter van Pijkeren is the founder and owner of the consulting company Next-Gen Probiotics, LLC. Other authors declare no conflict of interest, financial or otherwise.
Figures
Similar articles
-
Probiotic-Derived Ecto-5'-Nucleotidase Produces Anti-Inflammatory Adenosine Metabolites in Treg-Deficient Scurfy Mice.Probiotics Antimicrob Proteins. 2023 Aug;15(4):1001-1013. doi: 10.1007/s12602-023-10089-z. Epub 2023 May 13. Probiotics Antimicrob Proteins. 2023. PMID: 37178405 Free PMC article.
-
Evidence for the involvement of ecto-5'-nucleotidase (CD73) in drug resistance.Int J Cancer. 1996 Nov 15;68(4):493-500. doi: 10.1002/(SICI)1097-0215(19961115)68:4<493::AID-IJC15>3.0.CO;2-6. Int J Cancer. 1996. PMID: 8945621
-
Impairment of tubuloglomerular feedback regulation of GFR in ecto-5'-nucleotidase/CD73-deficient mice.J Clin Invest. 2004 Sep;114(5):634-42. doi: 10.1172/JCI21851. J Clin Invest. 2004. PMID: 15343381 Free PMC article.
-
The clinical biochemistry of 5'-nucleotidase.Ann Clin Lab Sci. 1990 Mar-Apr;20(2):123-39. Ann Clin Lab Sci. 1990. PMID: 2183704 Review.
-
Targeting ecto-5'-nucleotidase: A comprehensive review into small molecule inhibitors and expression modulators.Eur J Med Chem. 2023 Feb 5;247:115052. doi: 10.1016/j.ejmech.2022.115052. Epub 2022 Dec 30. Eur J Med Chem. 2023. PMID: 36599229 Review.
References
-
- Tan QKG, Louie RJ, Sleasman JW (1993) IPEX Syndrome. In: GeneReviews((R)), vol. (Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM et al., eds). Seattle (WA): https://www.ncbi.nlm.nih.gov/pubmed/20301297 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

