Modulus-dependent effects on neurogenic, myogenic, and chondrogenic differentiation of human mesenchymal stem cells in three-dimensional hydrogel cultures
- PMID: 37066837
- PMCID: PMC10935625
- DOI: 10.1002/jbm.a.37545
Modulus-dependent effects on neurogenic, myogenic, and chondrogenic differentiation of human mesenchymal stem cells in three-dimensional hydrogel cultures
Abstract
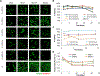
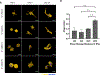
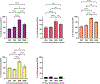
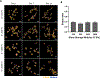
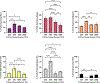
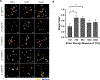
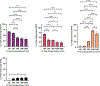
Human mesenchymal stromal cells (hMSCs) are of significant interest as a renewable source of therapeutically useful cells. In tissue engineering, hMSCs are implanted within a scaffold to provide enhanced capacity for tissue repair. The present study evaluates how mechanical properties of that scaffold can alter the phenotype and genotype of the cells, with the aim of augmenting hMSC differentiation along the myogenic, neurogenic or chondrogenic linages. The hMSCs were grown three-dimensionally (3D) in a hydrogel comprised of poly(ethylene glycol) (PEG)-conjugated to fibrinogen. The hydrogel's shear storage modulus (G'), which was controlled by increasing the amount of PEG-diacrylate cross-linker in the matrix, was varied in the range of 100-2000 Pascal (Pa). The differentiation into each lineage was initiated by a defined culture medium, and the hMSCs grown in the different modulus hydrogels were characterized using gene and protein expression. Materials having lower storage moduli (G' = 100 Pa) exhibited more hMSCs differentiating to neurogenic lineages. Myogenesis was favored in materials having intermediate modulus values (G' = 500 Pa), whereas chondrogenesis was favored in materials with a higher modulus (G' = 1000 Pa). Enhancing the differentiation pathway of hMSCs in 3D hydrogel scaffolds using simple modifications to mechanical properties represents an important achievement toward the effective application of these cells in tissue engineering.
Keywords: biomaterials; hydrogel; shear modulus; stem cells; tissue engineering.
© 2023 The Authors. Journal of Biomedical Materials Research Part A published by Wiley Periodicals LLC.
Figures
Similar articles
-
Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells.Acta Biomater. 2018 Jan 15;66:166-176. doi: 10.1016/j.actbio.2017.11.020. Epub 2017 Nov 8. Acta Biomater. 2018. PMID: 29128540
-
Generation of hyaline-like cartilage tissue from human mesenchymal stromal cells within the self-generated extracellular matrix.Acta Biomater. 2022 Sep 1;149:150-166. doi: 10.1016/j.actbio.2022.06.040. Epub 2022 Jun 30. Acta Biomater. 2022. PMID: 35779770
-
Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels.Acta Biomater. 2020 Mar 15;105:44-55. doi: 10.1016/j.actbio.2020.01.048. Epub 2020 Feb 5. Acta Biomater. 2020. PMID: 32035282
-
Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness.Acta Biomater. 2017 Aug;58:302-311. doi: 10.1016/j.actbio.2017.06.016. Epub 2017 Jun 10. Acta Biomater. 2017. PMID: 28611002 Free PMC article.
-
Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage.Biomaterials. 2010 Oct;31(28):7298-307. doi: 10.1016/j.biomaterials.2010.06.001. Biomaterials. 2010. PMID: 20615545
Cited by
-
The BAM-GelMA-ADSCs bilayer patch promotes tissue regeneration and functional recovery after large-area bladder defects in beagles.Bioeng Transl Med. 2025 Jan 15;10(3):e10745. doi: 10.1002/btm2.10745. eCollection 2025 May. Bioeng Transl Med. 2025. PMID: 40385542 Free PMC article.
-
Interlacing biology and engineering: An introduction to textiles and their application in tissue engineering.Mater Today Bio. 2025 Feb 25;31:101617. doi: 10.1016/j.mtbio.2025.101617. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124339 Free PMC article. Review.
References
-
- Ciocci M, Cacciotti I, Seliktar D, Melino S. Injectable silk fibroin hydrogels functionalized with microspheres as adult stem cells-carrier systems. International Journal of Biological Macromolecules 2018;108:960–971. - PubMed
-
- Birman T, Seliktar D. Injectability of Biosynthetic Hydrogels: Consideration for Minimally Invasive Surgical Procedures and 3D Bioprinting. Advanced Functional Materials 2021;31(29).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

