Method for estimating pulsatile wall shear stress from one-dimensional velocity waveforms
- PMID: 37066977
- PMCID: PMC10108024
- DOI: 10.14814/phy2.15628
Method for estimating pulsatile wall shear stress from one-dimensional velocity waveforms
Abstract
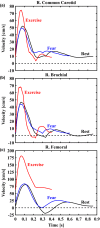
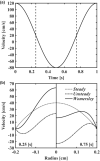
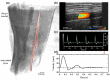
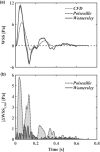
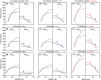
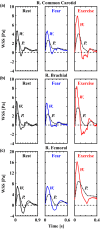
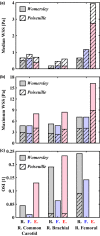
Wall shear stress (WSS)-a key regulator of endothelial function-is commonly estimated in vivo using simplified mathematical models based on Poiseuille's flow, assuming a quasi-steady parabolic velocity distribution, despite evidence that more rapidly time-varying, pulsatile blood flow during each cardiac cycle modulates flow-mediated dilation (FMD) in large arteries of healthy subjects. More exact and accurate models based on the well-established Womersley solution for rapidly changing blood flow have not been adopted clinically, potentially because the Womersley solution relies on the local pressure gradient, which is difficult to measure non-invasively. We have developed an open-source method for automatic reconstruction of unsteady, Womersley-derived velocity profiles, and WSS in conduit arteries. The proposed method (available online at https://doi.org/10.5281/zenodo.7576408) requires only the time-averaged diameter of the vessel and time-varying velocity data available from non-invasive imaging such as Doppler ultrasound. Validation of the method with subject-specific computational fluid dynamics and application to synthetic velocity waveforms in the common carotid, brachial, and femoral arteries reveals that the Poiseuille solution underestimates peak WSS 38.5%-55.1% during the acceleration and deceleration phases of systole and underestimates or neglects retrograde WSS. Following evidence that oscillatory shear significantly augments vasodilator production, it is plausible that mischaracterization of the shear stimulus by assuming parabolic flow leads to systematic underestimates of important biological effects of time-varying blood velocity in conduit arteries.
Keywords: Womersley solution; flow-mediated dilation; reduced-order model; shear rate; velocity profile.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Errors in the estimation of wall shear stress by maximum Doppler velocity.Atherosclerosis. 2013 Apr;227(2):259-66. doi: 10.1016/j.atherosclerosis.2013.01.026. Epub 2013 Jan 25. Atherosclerosis. 2013. PMID: 23398945 Free PMC article.
-
Effect of inlet velocity profiles on patient-specific computational fluid dynamics simulations of the carotid bifurcation.J Biomech Eng. 2012 May;134(5):051001. doi: 10.1115/1.4006681. J Biomech Eng. 2012. PMID: 22757489 Free PMC article.
-
Flow-based method demonstrates improved accuracy for calculating wall shear stress in arterial flows from 4D flow MRI data.J Biomech. 2023 Jan;146:111413. doi: 10.1016/j.jbiomech.2022.111413. Epub 2022 Dec 9. J Biomech. 2023. PMID: 36535100 Free PMC article.
-
Wall shear stress--an important determinant of endothelial cell function and structure--in the arterial system in vivo. Discrepancies with theory.J Vasc Res. 2006;43(3):251-69. doi: 10.1159/000091648. Epub 2006 Feb 20. J Vasc Res. 2006. PMID: 16491020 Review.
-
A Pseudo-Spectral Method for Wall Shear Stress Estimation from Doppler Ultrasound Imaging in Coronary Arteries.Cardiovasc Eng Technol. 2024 Dec;15(6):647-666. doi: 10.1007/s13239-024-00741-2. Epub 2024 Aug 5. Cardiovasc Eng Technol. 2024. PMID: 39103664 Review.
Cited by
-
Transport of nitrite from large arteries modulates regional blood flow during stress and exercise.Front Cardiovasc Med. 2023 Jun 12;10:1146717. doi: 10.3389/fcvm.2023.1146717. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37378407 Free PMC article.
References
-
- Ahrens, J. , Geveci, B. , & Law, C. (2005). ParaView: An end‐user tool for large‐data visualization. In Visualization handbook (pp. 717–731). Elsevier.
-
- Braith, R. W. , Conti, C. R. , Nichols, W. W. , Choi, C. Y. , Khuddus, M. A. , Beck, D. T. , & Casey, D. P. (2010). Enhanced external counterpulsation improves peripheral artery flow‐mediated dilation in patients with chronic angina. Circulation, 122, 1612–1620. 10.1161/CIRCULATIONAHA.109.923482 - DOI - PMC - PubMed
-
- Butler, P. J. , Weinbaum, S. , Chien, S. , & Lemons, D. E. (2000). Endothelium‐dependent, shear‐induced vasodilation is rate‐sensitive. Microcirculation, 7, 53–65. - PubMed
-
- Celermajer, D. S. , Sorensen, K. E. , Gooch, V. M. , Spiegelhalter, D. J. , Miller, O. I. , Sullivan, I. D. , Lloyd, J. K. , & Deanfield, J. E. (1992). Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet, 340, 1111–1115. 10.1016/0140-6736(92)93147-F - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

