Lipid peroxidation in diamond supported bilayers
- PMID: 37067002
- PMCID: PMC10273028
- DOI: 10.1039/d3nr01167d
Lipid peroxidation in diamond supported bilayers
Abstract
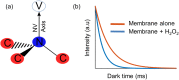
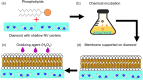
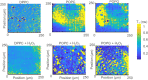
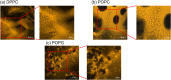
Lipid peroxidation is a process that occurs in cells when they are exposed to oxidative stress. During the process reactive oxygen species attack lipids within the lipid bilayers of cells. Since the products of lipid peroxidation are toxic and carcinogenic, it is important to understand where and how it occurs with nanoscale resolution. The radical intermediates of this process are particularly interesting since they are causing chain reactions damaging large parts of the lipid membranes in cells. However, they are also difficult to measure for the state of the art because they are short lived and reactive. Here, we study the lipid peroxidation of three artificial lipid bilayers on a diamonds substrate that can be used to study lipid peroxidation. In particular, we present a diamond quantum sensing method called T1-relaxometry that allows for in situ measurements and imaging of radical intermediates of lipid peroxidation in these membranes.
Conflict of interest statement
There are no conflicts to declare.
Figures




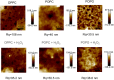
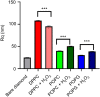
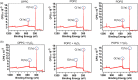

Similar articles
-
Lipid peroxidation of membrane phospholipids in the vertebrate retina.Front Biosci (Schol Ed). 2011 Jan 1;3(1):52-60. doi: 10.2741/s131. Front Biosci (Schol Ed). 2011. PMID: 21196356 Review.
-
Lipid peroxidation and tissue damage.In Vivo. 1999 May-Jun;13(3):295-309. In Vivo. 1999. PMID: 10459507 Review.
-
Appearance of homogeneous smectic multilamellar microenvironments in biomembranes undergoing superoxide-initiated lipid peroxidation: lipid-dienyl radical accumulation and fluidity management in lipid bilayers.Biochem Mol Biol Int. 1994 Aug;33(5):853-62. Biochem Mol Biol Int. 1994. PMID: 7987253
-
Determination of singlet oxygen-specific versus radical-mediated lipid peroxidation in photosensitized oxidation of lipid bilayers: effect of beta-carotene and alpha-tocopherol.Biochemistry. 1997 Oct 21;36(42):12911-20. doi: 10.1021/bi9708646. Biochemistry. 1997. PMID: 9335550
-
Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives.J Biol Chem. 1994 Oct 21;269(42):26066-75. J Biol Chem. 1994. PMID: 7929318
Cited by
-
Diamond Surfaces with Lateral Gradients for Systematic Optimization of Surface Chemistry for Relaxometry - a Low-Pressure Plasma-Based Approach.Langmuir. 2024 Oct 29;40(43):23007-23017. doi: 10.1021/acs.langmuir.4c03171. Epub 2024 Oct 18. Langmuir. 2024. PMID: 39421905 Free PMC article.
References
-
- Retamal M. J. Cisternas M. A. Gutierrez-Maldonado S. E. Perez-Acle T. Seifert B. Busch M. Volkmann U. G. Towards bio-silicon interfaces: Formation of an ultra-thin self-hydrated artificial membrane composed of dipalmitoylphosphatidylcholine (DPPC) and chitosan deposited in high vacuum from the gas-phase. J. Chem. Phys. 2014;141(10):09B604_1. doi: 10.1063/1.4894224. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

