Melanoma stem cell vaccine induces effective tumor immunity against melanoma
- PMID: 37067182
- PMCID: PMC10114997
- DOI: 10.1080/21645515.2022.2158670
Melanoma stem cell vaccine induces effective tumor immunity against melanoma
Abstract
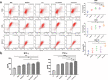
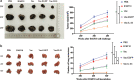
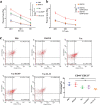
Melanoma stem cells (MSCs)-based vaccine strategies have been a potent immunotherapeutic approach for melanoma treatment, which aimed at inducing specific anti-tumor immunity and targeting cancer stem-like cells. As the main cancer-fighting immune cells, CD8+T cells play an important role in vaccine-induced antitumor immunity. Here, we developed a novel MSC vaccine that induces CD8+T cells to target melanoma stem cells specifically. The MSC vaccine was prepared for our study in order to determine the effectiveness of antitumor immunity. The proportion and activity of CD8+T cells were examined in the spleen after immunization, in particular, the expression and cytotoxicity of the immune checkpoint of spleen lymphocytes were detected by flow cytometry and ELISA, moreover, tumor size and the number of lung metastasis nodules were observed and the specific killing effect of the vaccine was evaluated in immunized mice. We found that the MSC vaccine could promote DCs maturation, activate CD8+T cells, suppress the expression of CTLA-4, PD-1, and Tim-3, and increase the expression of IFN-γ and GzmB of CD8+T cells. Melanoma growth and metastasis were inhibited by the vaccine's specific targeted killing effect. The vaccines based on melanoma stem cells (MSCs) delay the progression of melanoma by inducing anti-tumor immune responses in CD8+T cells.
Keywords: CD8+T cells; Melanoma stem cell; immune checkpoints; melanoma; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Albittar AA, Alhalabi O, Glitza Oliva IC. Immunotherapy for melanoma. Adv Exp Med Biol. 2020;1244:51–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
