Feasibility of redo-TAVI in self-expanding Evolut valves: a CT analysis from the Evolut Low Risk Trial substudy
- PMID: 37067193
- PMCID: PMC10333920
- DOI: 10.4244/EIJ-D-22-01125
Feasibility of redo-TAVI in self-expanding Evolut valves: a CT analysis from the Evolut Low Risk Trial substudy
Abstract
Background: Transcatheter aortic valve implantation in an existing transcatheter valve (redo-TAVI) pins the index valve leaflets in the open position (neoskirt), which can cause coronary flow compromise and limit access. Whether anatomy may preclude redo-TAVI in self-expanding Evolut valves is unknown.
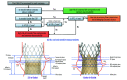
Aims: We aimed to evaluate the anatomical feasibility of redo-TAVI by simulating implantation of a balloon-expandable SAPIEN 3 (S3) within an Evolut or an Evolut within an Evolut.
Methods: A total of 204 post-TAVI computed tomography (CT) scans from the Evolut Low Risk CT substudy were analysed. Five redo-TAVI positions were evaluated: S3-in-Evolut inflow-to-inflow, S3 outflow at Evolut nodes 4, 5, and 6, and Evolut-in-Evolut inflow-to-inflow. Univariable modelling identified pre-TAVI clinical characteristics, CT anatomical parameters, and procedural variables associated with coronary flow compromise using the neoskirt height and post-TAVI aortic root dimensions.
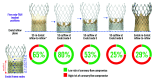
Results: The risk of coronary flow compromise was lowest when the S3 outflow was at Evolut node 4 (20%) and highest when at Evolut node 6 (75%). The highest likelihood of preserving coronary accessibility occurred with the S3 outflow at Evolut node 4. Female sex and higher body mass index were associated with a higher risk of coronary flow compromise, as were a smaller annulus diameter, lower sinus of Valsalva height and width, shorter coronary height, smaller sinotubular junction diameter, and shallower Evolut implant depth.
Conclusions: The feasibility of redo-TAVI after Evolut failure is multifactorial and relates to the native annular anatomy, as well as the implantation depth of the index and second bioprostheses. Placement of an S3 at a lower Evolut position may reduce the risk of coronary flow compromise while preserving coronary access.
Clinicaltrials: gov: NCT02701283.
Conflict of interest statement
K.J.Grubb is aproctor, principal investigator and serves on the advisory board for Medtronic; and also serves on the advisory board or is aconsultant for Ancora Heart, Boston Scientific, Abbott, 4C Medical, and Edwards Lifesciences. J.Spencer is an employee and shareholder of Medtronic. G.H.L.Tang is aphysician proctor and consultant for Medtronic, aconsultant and physician advisory board member for Abbott Structural Heart, and aphysician advisory board member for JenaValve. J.Zhingre Sanchez is an employee and shareholder of Medtronic. J.Sathananthan is aconsultant to Edwards Lifesciences and Medtronic; and has received speaker fees from Edwards Lifesciences and NVT.T.Rogers is aconsultant and physician proctor to Edwards Lifesciences, Medtronic, and Boston Scientific; is an advisory board member to Medtronic; has equity in Transmural Systems; and is aco-inventor on patents, assigned to NIH, for transcatheter electrosurgery devices. M.Deeb serves on an advisory board for Medtronic; and has received institutional grant support from Boston Scientific, Edwards Lifesciences, and Medtronic; he receives no personal remunerations. S.Fukuhara is aconsultant for Terumo Aortic, Medtronic, and Artivion. P.Blanke holds institutional research core lab agreements with Medtronic, Edwards Lifesciences, and Abbott, with no personal compensation. J.A.Leipsic holds institutional research core lab agreements with Medtronic, Edwards Lifesciences, Abbott, Boston Scientific, and Pi-Cardia, with no personal compensation. J.K.Forrest has received grant support/research contracts and consultant fees/honoraria/speakers’ bureau fees from Edwards Lifesciences and Medtronic. M.J.Reardon has received fees to his institution from Medtronic for consulting and providing educational services. P.Gleason’s employer receives institutional grants and educational funding from Edwards Lifesciences and Medtronic; he has no personal financial disclosures. The other authors have no conflicts of interest relevant to this paper to declare.
Figures

References
-
- Writing Committee Members Otto C, Nishimura R, Bonow R, Carabello B, Erwin JP 3rd, Gentile F, Jneid H, Krieger E V, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt T 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:450–500. - PubMed
-
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. - PMC - PubMed
-
- Sharma T, Krishnan AM, Lahoud R, Polomsky M, Dauerman H. National Trends in TAVR and SAVR for Patients With Severe Isolated Aortic Stenosis. J Am Coll Cardiol. 2022;80:2054–56. - PubMed
-
- O’Hair D, Yakubov SJ, Oh JK, Ito S, Deeb GM, Van Mieghem, Adams DH, Bajwa T, Kleiman NS, Chetcuti S, Søndergaard L, Gada H, Mumtaz M, Heiser J, Merhi WM, Petrossian G, Robinson N, Tang GHL, Rovin JD, Little SH, Jain R, Verdoliva S, Hanson T, Li S, Popma JJ, Reardon MJ. Structural Valve Deterioration After Self-Expanding Transcatheter or Surgical Aortic Valve Implantation in Patients at Intermediate or High Risk. JAMA Cardiol. 2023;8:111–9. - PMC - PubMed
-
- Landes U, Richter I, Danenberg H, Kornowski R, Sathananthan J, De Backer, Søndergaard L, Abdel-Wahab M, Yoon SH, Makkar RR, Thiele H, Kim WK, Hamm C, Buzzatti N, Montorfano M, Ludwig S, Schofer N, Voigtlaender L, Guerrero M, El Sabbagh, Rodés-Cabau J, Mesnier J, Okuno T, Pilgrim T, Fiorina C, Colombo A, Mangieri A, Eltchaninoff H, Nombela-Franco L, Van Wiechen, Van Mieghem, Tchétché D, Schoels WH, Kullmer M, Barbanti M, Tamburino C, Sinning JM, Al-Kassou B, Perlman GY, Ielasi A, Fraccaro C, Tarantini G, De Marco, Witberg G, Redwood SR, Lisko JC, Babaliaros VC, Laine M, Nerla R, Finkelstein A, Eitan A, Jaffe R, Ruile P, Neumann FJ, Piazza N, Sievert H, Sievert K, Russo M, Andreas M, Bunc M, Latib A, Bruoha S, Godfrey R, Hildick-Smith D, Barbash I, Segev A, Maurovich-Horvat P, Szilveszter B, Spargias K, Aravadinos D, Nazif TM, Leon MB, Webb JG. Outcomes of Redo Transcatheter Aortic Valve Replacement According to the Initial and Subsequent Valve Type. JACC Cardiovasc Interv. 2022;15:1543–54. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

