BZW2 Inhibition Reduces Colorectal Cancer Growth and Metastasis
- PMID: 37067340
- PMCID: PMC10329991
- DOI: 10.1158/1541-7786.MCR-23-0003
BZW2 Inhibition Reduces Colorectal Cancer Growth and Metastasis
Abstract
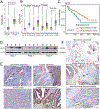
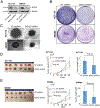
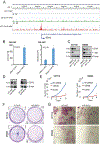
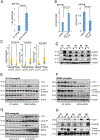
Because survival of patients with metastatic colorectal cancer remain poor, there is an urgent need to identify potential novel druggable targets that are associated with colorectal cancer progression. One such target, basic leucine zipper and W2 domains 2 (BZW2), is involved in regulation of protein translation, and its overexpression is associated with human malignancy. Thus, we investigated the expression and regulation of BZW2, assessed its role in activation of WNT/β-catenin signaling, identified its downstream molecules, and demonstrated its involvement in metastasis of colorectal cancer. In human colorectal cancers, high mRNA and protein expression levels of BZW2 were associated with tumor progression. BZW2-knockdown reduced malignant phenotypes, including cell proliferation, invasion, and spheroid and colony formation. BZW2-knockdown also reduced tumor growth and metastasis; conversely, transfection of BZW2 into BZW2 low-expressing colorectal cancer cells promoted malignant features, including tumor growth and metastasis. BZW2 expression was coordinately regulated by microRNA-98, c-Myc, and histone methyltransferase enhancer of zeste homolog 2 (EZH2). RNA sequencing analyses of colorectal cancer cells modulated for BZW2 identified P4HA1 and the long noncoding RNAs, MALAT1 and NEAT1, as its downstream targets. Further, BZW2 activated the Wnt/β-catenin signaling pathway in colorectal cancers expressing wild-type β-catenin. In sum, our study suggests the possibility of targeting BZW2 expression by inhibiting EZH2 and/or c-Myc.
Implications: FDA-approved small-molecule inhibitors of EZH2 can indirectly target BZW2 and because BZW2 functions as an oncogene, these inhibitors could serve as therapeutic agents for colorectal cancer.
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

