Contact Guidance of Connective Tissue Fibroblasts on Submicrometer Anisotropic Topographical Cues Is Dependent on Tissue of Origin, β1 Integrins, and Tensin-1 Recruitment
- PMID: 37067372
- PMCID: PMC10141244
- DOI: 10.1021/acsami.2c22381
Contact Guidance of Connective Tissue Fibroblasts on Submicrometer Anisotropic Topographical Cues Is Dependent on Tissue of Origin, β1 Integrins, and Tensin-1 Recruitment
Abstract
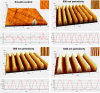
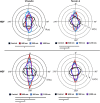
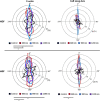
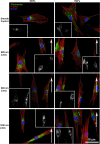
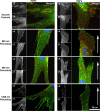
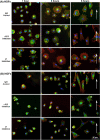
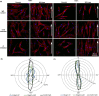
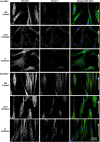
The substratum topography of both natural and synthetic materials is a prominent regulator of cell behaviors including adhesion, migration, matrix fibrillogenesis, and cell phenotype. Connective tissue fibroblasts are known to respond to repeating groove topographical modifications by aligning and exhibiting directed migration, a phenomenon termed contact guidance. Although both reside in collagen rich connective tissues, dermal and gingival fibroblasts are known to exhibit differences in phenotype during wound healing, with gingival tissue showing a fetal-like scarless response. Differences in adhesion formation and maturation are known to underlie both a scarring phenotype and cell response to topographical features. Utilizing repeating groove substrates with periodicities of 600, 900, and 1200 nm (depth, 100 nm), we investigated the roles of integrins αvβ3 and β1 associated adhesions on contact guidance of human gingival (HGFs) and dermal fibroblasts (HDFs). HGFs showed a higher degree of orientation with the groove long axis than HDFs, with alignment of both vinculin and tensin-1 evident on 600 and 900 nm periodicities in both cell types. Orientation with grooves of any periodicity in HGFs and HDFs did not alter the adhesion number or area compared to smooth control surfaces. Growth of both cell types on all periodicities reduced fibronectin fibrillogenesis compared to control surfaces. Independent inhibition of integrin αvβ3 and β1 in both cell types induced changes in spreading up to 6 h and reduced alignment with the groove long axis. At 24 h post-seeding with blocking antibodies, HGFs recovered orientation, but in HDFs, blocking of β1, but not αvβ3 integrins, inhibited alignment. Blocking of β1 and αvβ3 in HDFs, but not HGFs, inhibited tensin-1-associated fibrillar adhesion formation. Furthermore, inhibition of β1 integrins in HDFs, but not HGFs, resulted in recruitment of tensin-1 to αvβ3 focal adhesions, preventing HDFs from aligning with the groove long axis. Our work demonstrates that tensin-1 localization with specific integrins in adhesion sites is an important determinant of contact guidance. This work emphasizes further the need for tissue-specific biomaterials, when integration into host tissues is required.
Keywords: directed migration; fibrillar adhesion; fibroblasts; submicrometer topography; wound healing.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis.J Cell Biol. 2002 Dec 23;159(6):1071-86. doi: 10.1083/jcb.200205014. Epub 2002 Dec 16. J Cell Biol. 2002. PMID: 12486108 Free PMC article.
-
AMPK negatively regulates tensin-dependent integrin activity.J Cell Biol. 2017 Apr 3;216(4):1107-1121. doi: 10.1083/jcb.201609066. Epub 2017 Mar 13. J Cell Biol. 2017. PMID: 28289092 Free PMC article.
-
Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation.Biol Cell. 2007 Nov;99(11):601-14. doi: 10.1042/BC20070008. Biol Cell. 2007. PMID: 17516912
-
Tensins: Bridging AMP-Activated Protein Kinase with Integrin Activation.Trends Cell Biol. 2017 Oct;27(10):703-711. doi: 10.1016/j.tcb.2017.06.004. Epub 2017 Jul 8. Trends Cell Biol. 2017. PMID: 28698049 Review.
-
Hiding in Plain Sight: Human Gingival Fibroblasts as an Essential, Yet Overlooked, Tool in Regenerative Medicine.Cells. 2023 Aug 8;12(16):2021. doi: 10.3390/cells12162021. Cells. 2023. PMID: 37626831 Free PMC article. Review.
Cited by
-
Merging Modular Molecular Design with High Throughput Screening of Cell Adhesion on Antimicrobial Supramolecular Biomaterials.Macromol Rapid Commun. 2025 Apr;46(8):e2300638. doi: 10.1002/marc.202300638. Epub 2024 Apr 13. Macromol Rapid Commun. 2025. PMID: 38530968 Free PMC article.
-
Galectin-3/Gelatin Electrospun Scaffolds Modulate Collagen Synthesis in Skin Healing but Do Not Improve Wound Closure Kinetics.Bioengineering (Basel). 2024 Sep 25;11(10):960. doi: 10.3390/bioengineering11100960. Bioengineering (Basel). 2024. PMID: 39451336 Free PMC article.
-
Dimensionality Matters: Exploiting UV-Photopatterned 2D and Two-Photon-Printed 2.5D Contact Guidance Cues to Control Corneal Fibroblast Behavior and Collagen Deposition.Bioengineering (Basel). 2024 Apr 19;11(4):402. doi: 10.3390/bioengineering11040402. Bioengineering (Basel). 2024. PMID: 38671823 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

