Influence of Maternal Breast Milk and Vaginal Microbiome on Neonatal Gut Microbiome: a Longitudinal Study during the First Year
- PMID: 37067419
- PMCID: PMC10269640
- DOI: 10.1128/spectrum.04967-22
Influence of Maternal Breast Milk and Vaginal Microbiome on Neonatal Gut Microbiome: a Longitudinal Study during the First Year
Abstract
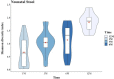
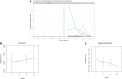
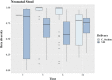
It is believed that establishment of the gut microbiome starts very early in life and is crucial for growth, immunity, and long-term metabolic health. In this longitudinal study, we recruited 25 mothers in their third trimester, of whom 15 had vaginal delivery while 10 had an unplanned cesarean section (C-section). The mother-neonate pairs were followed for 1 year, and we generated 16S metagenomic data to study the neonatal gut microbiome along with mother's breast milk and vaginal microbiomes through 12 months after delivery, at 1, 3, 6, and 12 months. We inferred (i) mode of delivery is an important factor influencing both composition and entropy of the neonatal gut microbiome, and the genus Streptococcus plays an important role in the temporal differentiation. (ii) Microbial diversity monotonically increases with age, irrespective of the mode of delivery, and it is significantly altered once exclusive breastfeeding is stopped. (iii) We found little evidence in favor of the microflora of mother's breast milk and a vaginal swab being directly reflected in the offspring's gut microbiome; however, some distinction could be made in the gut microbiome of neonates whose mothers were classified as community state type III (CSTIII) and CSTIV, based on their vaginal microbiomes. (iv) A lot of the mature gut microbiome is possibly acquired from the environment, as the genera Prevotella and Faecalibacterium, two of the most abundant flora in the neonatal gut microbiome, are introduced after initiation of solidified food. The distinction between the gut microbiome of babies born by vaginal delivery and babies born by C-section becomes blurred after introduction of solid food, although the diversity in the gut microbiota drastically increases in both cases. IMPORTANCE Gut microbiome architecture seems to have a potential impact on host metabolism, health, and nutrition. Early life gut microbiome development is considered a crucial phenomenon for neonatal health as well as adulthood metabolic complications. In this longitudinal study, we examined the association of neonatal gut microbiome entropy and its temporal variation. The study revealed that adult-like gut microbiome architecture starts taking shape after initiation of solidified food. Further, we also observed that the difference of microbial diversity was reduced between vaginally delivered and C-section babies compared to exclusive breastfeeding tenure. We found evidence in favor of the inheritance of the microflora of mother's posterior vaginal wall to the offspring's gut microbiome.
Keywords: breast milk; gut microbiome; maternal microbiome; neonatal; neonatal stool.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome.JAMA Pediatr. 2017 Jul 1;171(7):647-654. doi: 10.1001/jamapediatrics.2017.0378. JAMA Pediatr. 2017. PMID: 28492938 Free PMC article.
-
Comparison of Gut Microbiomes Between Neonates Born by Cesarean Section and Vaginal Delivery: Prospective Observational Study.Biomed Res Int. 2024 Nov 28;2024:8302361. doi: 10.1155/bmri/8302361. eCollection 2024. Biomed Res Int. 2024. PMID: 39640900 Free PMC article.
-
Birth Mode Does Not Determine the Presence of Shared Bacterial Strains between the Maternal Vaginal Microbiome and the Infant Stool Microbiome.Microbiol Spectr. 2023 Aug 17;11(4):e0061423. doi: 10.1128/spectrum.00614-23. Epub 2023 Jun 20. Microbiol Spectr. 2023. PMID: 37338388 Free PMC article.
-
Unveiling the neonatal gut microbiota: exploring the influence of delivery mode on early microbial colonization and intervention strategies.Arch Gynecol Obstet. 2024 Dec;310(6):2853-2861. doi: 10.1007/s00404-024-07843-1. Epub 2024 Nov 26. Arch Gynecol Obstet. 2024. PMID: 39589476 Review.
-
Gut Microbiota Composition in Healthy Japanese Infants and Young Adults Born by C-Section.Ann Nutr Metab. 2018;73 Suppl 3:4-11. doi: 10.1159/000490841. Epub 2018 Jul 24. Ann Nutr Metab. 2018. PMID: 30041174 Review.
Cited by
-
Complementary Feeding and Infant Gut Microbiota: A Narrative Review.Nutrients. 2025 Feb 20;17(5):743. doi: 10.3390/nu17050743. Nutrients. 2025. PMID: 40077613 Free PMC article. Review.
-
The Neonatal Microbiome: Implications for Amyotrophic Lateral Sclerosis and Other Neurodegenerations.Brain Sci. 2025 Feb 14;15(2):195. doi: 10.3390/brainsci15020195. Brain Sci. 2025. PMID: 40002527 Free PMC article. Review.
-
Maternal Psychological Well-Being as a Protector in Infantile Colic.Nutrients. 2024 Jul 19;16(14):2342. doi: 10.3390/nu16142342. Nutrients. 2024. PMID: 39064784 Free PMC article.
-
Vaginal microbiota transplantation is a truly opulent and promising edge: fully grasp its potential.Front Cell Infect Microbiol. 2024 Mar 22;14:1280636. doi: 10.3389/fcimb.2024.1280636. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38585656 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

