Impact of Wood Age on Termite Microbial Assemblages
- PMID: 37067424
- PMCID: PMC10231148
- DOI: 10.1128/aem.00361-23
Impact of Wood Age on Termite Microbial Assemblages
Abstract
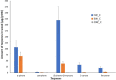
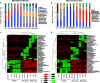
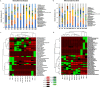
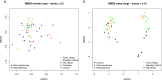
The decomposition of wood and detritus is challenging to most macroscopic organisms due to the recalcitrant nature of lignocellulose. Moreover, woody plants often protect themselves by synthesizing toxic or nocent compounds which infuse their tissues. Termites are essential wood decomposers in warmer terrestrial ecosystems and, as such, they have to cope with high concentrations of plant toxins in wood. In this paper, we evaluated the influence of wood age on the gut microbial (bacterial and fungal) communities associated with the termites Reticulitermes flavipes (Rhinotermitidae) (Kollar, 1837) and Microcerotermes biroi (Termitidae) (Desneux, 1905). We confirmed that the secondary metabolite concentration decreased with wood age. We identified a core microbial consortium maintained in the gut of R. flavipes and M. biroi and found that its diversity and composition were not altered by the wood age. Therefore, the concentration of secondary metabolites had no effect on the termite gut microbiome. We also found that both termite feeding activities and wood age affect the wood microbiome. Whether the increasing relative abundance of microbes with termite activities is beneficial to the termites is unknown and remains to be investigated. IMPORTANCE Termites can feed on wood thanks to their association with their gut microbes. However, the current understanding of termites as holobiont is limited. To our knowledge, no studies comprehensively reveal the influence of wood age on the termite-associated microbial assemblage. The wood of many tree species contains high concentrations of plant toxins that can vary with their age and may influence microbes. Here, we studied the impact of Norway spruce wood of varying ages and terpene concentrations on the microbial communities associated with the termites Reticulitermes flavipes (Rhinotermitidae) and Microcerotermes biroi (Termitidae). We performed a bacterial 16S rRNA and fungal ITS2 metabarcoding study to reveal the microbial communities associated with R. flavipes and M. biroi and their impact on shaping the wood microbiome. We noted that a stable core microbiome in the termites was unaltered by the feeding substrate, while termite activities influenced the wood microbiome, suggesting that plant secondary metabolites have negligible effects on the termite gut microbiome. Hence, our study shed new insights into the termite-associated microbial assemblage under the influence of varying amounts of terpene content in wood and provides a groundwork for future investigations for developing symbiont-mediated termite control measures.
Keywords: Microcerotermes biroi; Reticulitermes flavipes; bacteria; core-microbiome; ectosymbionts; endosymbionts; fungi; plant defenses; terpenoids; wood-feeding termites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

