Steric Effects on the Chelation of Mn2+ and Zn2+ by Hexadentate Polyimidazole Ligands: Modeling Metal Binding by Calprotectin Site 2
- PMID: 37067464
- PMCID: PMC10640917
- DOI: 10.1002/chem.202300447
Steric Effects on the Chelation of Mn2+ and Zn2+ by Hexadentate Polyimidazole Ligands: Modeling Metal Binding by Calprotectin Site 2
Abstract
Recently, there has been increasing interest in the design of ligands that bind Mn2+ with high affinity and selectivity, but this remains a difficult challenge. It has been proposed that the cavity size of the binding pocket is a critical factor in most synthetic and biological examples of selective Mn2+ binding. Here, we use a bioinspired approach adapted from the hexahistidine binding site of the manganese-sequestering protein calprotectin to systematically study the effect of cavity size on Mn2+ and Zn2+ binding. We have designed a hexadentate, trisimidazole ligand whose cavity size can be tuned through peripheral modification of the steric bulk of the imidazole substituents. Conformational dynamics and redox potentials of the complexes are dependent on ligand steric bulk. Stability constants are consistent with the hypothesis that larger ligand cavities are relatively favorable for Mn2+ over Zn2+ , but this effect alone may not be sufficient to achieve Mn2+ selectivity.
Keywords: bioinorganic chemistry; chelates; manganese; metalloprotein mimcs; steric effects.
© 2023 Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of Interest
There are no conflicts to declare.
Figures

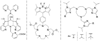
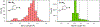
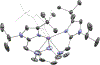
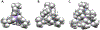
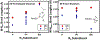
 , Mn2+) and blue diamonds (
, Mn2+) and blue diamonds ( , Zn2+) correspond to crystallographically determined bond distances.
, Zn2+) correspond to crystallographically determined bond distances.
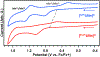
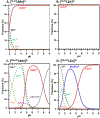

Similar articles
-
High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface.J Am Chem Soc. 2013 Jan 16;135(2):775-87. doi: 10.1021/ja3096416. Epub 2012 Dec 31. J Am Chem Soc. 2013. PMID: 23276281 Free PMC article.
-
Murine Calprotectin Coordinates Mn(II) at a Hexahistidine Site with Ca(II)-Dependent Affinity.Inorg Chem. 2019 Oct 21;58(20):13578-13590. doi: 10.1021/acs.inorgchem.9b00763. Epub 2019 May 30. Inorg Chem. 2019. PMID: 31145609 Free PMC article.
-
Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis.J Am Chem Soc. 2015 Mar 4;137(8):3004-16. doi: 10.1021/ja512204s. Epub 2015 Feb 18. J Am Chem Soc. 2015. PMID: 25597447 Free PMC article.
-
Synthetic Advances for Mechanistic Insights: Metal-Oxygen Intermediates with a Macrocyclic Pyridinophane System.Acc Chem Res. 2024 Jan 2;57(1):120-130. doi: 10.1021/acs.accounts.3c00582. Epub 2023 Dec 18. Acc Chem Res. 2024. PMID: 38110355
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
Cited by
-
Synthesis and Characterization of Bipyridyl-(Imidazole)n Mn(II) Compounds and Their Evaluation as Potential Precatalysts for Water Oxidation.Molecules. 2023 Oct 23;28(20):7221. doi: 10.3390/molecules28207221. Molecules. 2023. PMID: 37894706 Free PMC article.
References
-
- Waldron KJ, Robinson NJ, Nat. Rev. Microbiol 2009, 7, 25–35. - PubMed
-
- Bakthavatsalam S, Sarkar A, Rakshit A, Jain S, Kumar A, Datta A, Chem. Commun 2015, 51, 2605–2608. - PubMed
-
- Das S, Khatua K, Rakshit A, Carmona A, Sarkar A, Bakthavatsalam S, Ortega R, Datta A, Dalton Trans. 2019, 48, 7047–7061. - PubMed
-
- Drahoš B, Lukeš I, Tóth É, Eur. J. Inorg. Chem 2012, 2012, 1975–1986.
Grants and funding
LinkOut - more resources
Full Text Sources

