Using Cluster Theory to Calculate the Experimental Structure Factors of Antibody Solutions
- PMID: 37067466
- PMCID: PMC10155212
- DOI: 10.1021/acs.molpharmaceut.3c00191
Using Cluster Theory to Calculate the Experimental Structure Factors of Antibody Solutions
Abstract
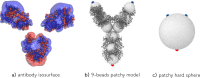
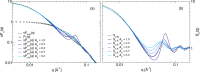
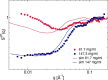
Monoclonal antibody solutions are set to become a major therapeutic tool in the years to come, capable of targeting various diseases by clever design of their antigen binding site. However, the formulation of stable solutions suitable for patient self-administration typically presents challenges, as a result of the increase in viscosity that often occurs at high concentrations. Here, we establish a link between the microscopic molecular details and the resulting properties of an antibody solution through the characterization of clusters, which arise in the presence of self-associating antibodies. In particular, we find that experimental small-angle X-ray scattering data can be interpreted by means of analytical models previously exploited for the study of polymeric and colloidal objects, based on the presence of such clusters. The latter are determined by theoretical calculations and supported by computer simulations of a coarse-grained minimal model, in which antibodies are treated as Y-shaped colloidal molecules and attractive domains are designed as patches. Using the theoretically predicted cluster size distributions, we are able to describe the experimental structure factors over a wide range of concentration and salt conditions. We thus provide microscopic evidence for the well-established fact that the concentration-dependent increase in viscosity is originated by the presence of clusters. Our findings bring new insights on the self-assembly of monoclonal antibodies, which can be exploited for guiding the formulation of stable and effective antibody solutions.
Keywords: Monte Carlo simulations; antibodies; cluster theory; colloids; patchy models; small-angle X-ray scattering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









Similar articles
-
Characterizing Protein-Protein Interactions and Viscosity of a Monoclonal Antibody from Low to High Concentration Using Small-Angle X-ray Scattering and Molecular Dynamics Simulations.Mol Pharm. 2023 Nov 6;20(11):5563-5578. doi: 10.1021/acs.molpharmaceut.3c00484. Epub 2023 Oct 2. Mol Pharm. 2023. PMID: 37782765
-
X-ray Scattering and Coarse-Grained Simulations for Clustering and Interactions of Monoclonal Antibodies at High Concentrations.J Phys Chem B. 2019 Jun 27;123(25):5274-5290. doi: 10.1021/acs.jpcb.9b04478. Epub 2019 Jun 18. J Phys Chem B. 2019. PMID: 31146525
-
Coarse-Grained Molecular Dynamics Simulations for Understanding the Impact of Short-Range Anisotropic Attractions on Structure and Viscosity of Concentrated Monoclonal Antibody Solutions.Mol Pharm. 2020 May 4;17(5):1748-1756. doi: 10.1021/acs.molpharmaceut.9b00960. Epub 2020 Apr 2. Mol Pharm. 2020. PMID: 32101441
-
Characterizing Experimental Monoclonal Antibody Interactions and Clustering Using a Coarse-Grained Simulation Library and a Viscosity Model.J Phys Chem B. 2023 Feb 9;127(5):1120-1137. doi: 10.1021/acs.jpcb.2c07616. Epub 2023 Jan 30. J Phys Chem B. 2023. PMID: 36716270
-
Improving monoclonal antibody selection and engineering using measurements of colloidal protein interactions.J Pharm Sci. 2014 Nov;103(11):3356-3363. doi: 10.1002/jps.24130. Epub 2014 Sep 10. J Pharm Sci. 2014. PMID: 25209466 Free PMC article. Review.
Cited by
-
Poly(glutamic acid)-Based Viscosity Reducers for Concentrated Formulations of a Monoclonal IgG Antibody.Mol Pharm. 2024 Feb 5;21(2):982-991. doi: 10.1021/acs.molpharmaceut.3c01159. Epub 2024 Jan 19. Mol Pharm. 2024. PMID: 38240032 Free PMC article.
-
Growth of Clusters toward Liquid-Liquid Phase Separation of Monoclonal Antibodies as Characterized by Small-Angle X-ray Scattering and Molecular Dynamics Simulation.J Phys Chem B. 2025 Mar 20;129(11):2856-2871. doi: 10.1021/acs.jpcb.4c07064. Epub 2025 Mar 7. J Phys Chem B. 2025. PMID: 40053704
-
A multi-scale numerical approach to study monoclonal antibodies in solution.APL Bioeng. 2024 Feb 26;8(1):016111. doi: 10.1063/5.0186642. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38425712 Free PMC article.
-
Combining Scattering Experiments and Colloid Theory to Characterize Charge Effects in Concentrated Antibody Solutions.Mol Pharm. 2024 May 6;21(5):2250-2271. doi: 10.1021/acs.molpharmaceut.3c01023. Epub 2024 Apr 25. Mol Pharm. 2024. PMID: 38661388 Free PMC article.
-
Reconciling predicted and measured viscosity parameters in high concentration therapeutic antibody solutions.MAbs. 2024 Jan-Dec;16(1):2438172. doi: 10.1080/19420862.2024.2438172. Epub 2024 Dec 11. MAbs. 2024. PMID: 39663541 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

