Translatome analysis reveals microglia and astrocytes to be distinct regulators of inflammation in the hyperacute and acute phases after stroke
- PMID: 37067534
- PMCID: PMC10330240
- DOI: 10.1002/glia.24377
Translatome analysis reveals microglia and astrocytes to be distinct regulators of inflammation in the hyperacute and acute phases after stroke
Abstract
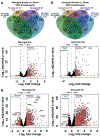
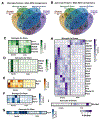
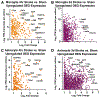
Neuroinflammation is a hallmark of ischemic stroke, which is a leading cause of death and long-term disability. Understanding the exact cellular signaling pathways that initiate and propagate neuroinflammation after stroke will be critical for developing immunomodulatory stroke therapies. In particular, the precise mechanisms of inflammatory signaling in the clinically relevant hyperacute period, hours after stroke, have not been elucidated. We used the RiboTag technique to obtain microglia and astrocyte-derived mRNA transcripts in a hyperacute (4 h) and acute (3 days) period after stroke, as these two cell types are key modulators of acute neuroinflammation. Microglia initiated a rapid response to stroke at 4 h by adopting an inflammatory profile associated with the recruitment of immune cells. The hyperacute astrocyte profile was marked by stress response genes and transcription factors, such as Fos and Jun, involved in pro-inflammatory pathways such as TNF-α. By 3 days, microglia shift to a proliferative state and astrocytes strengthen their inflammatory response. The astrocyte pro-inflammatory response at 3 days is partially driven by the upregulation of the transcription factors C/EBPβ, Spi1, and Rel, which comprise 25% of upregulated transcription factor-target interactions. Surprisingly, few sex differences across all groups were observed. Expression and log2 fold data for all sequenced genes are available on a user-friendly website for researchers to examine gene changes and generate hypotheses for stroke targets. Taken together, our data comprehensively describe the microglia and astrocyte-specific translatome response in the hyperacute and acute period after stroke and identify pathways critical for initiating neuroinflammation.
Keywords: RNASeq; ShinyApp; glia; inflammation; transcription factor.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Figures






Update of
-
Translatome analysis reveals microglia and astrocytes to be distinct regulators of inflammation in the hyperacute and acute phases after stroke.bioRxiv [Preprint]. 2023 Feb 17:2023.02.14.520351. doi: 10.1101/2023.02.14.520351. bioRxiv. 2023. Update in: Glia. 2023 Aug;71(8):1960-1984. doi: 10.1002/glia.24377. PMID: 36824949 Free PMC article. Updated. Preprint.
References
-
- Aleyasin Hossein, Cregan Sean P., Iyirhiaro Grace, O’Hare Michael J., Callaghan Steve M., Slack Ruth S., and Park David S.. 2004. “Nuclear Factor-(kappa)B Modulates the p53 Response in Neurons Exposed to DNA Damage.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 24 (12): 2963–73. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

