The contribution of N-terminal truncated cMyBPC to in vivo cardiac function
- PMID: 37067542
- PMCID: PMC10114924
- DOI: 10.1085/jgp.202213318
The contribution of N-terminal truncated cMyBPC to in vivo cardiac function
Abstract
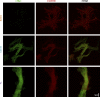
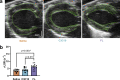
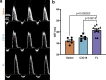
Cardiac myosin binding protein C (cMyBPC) is an 11-domain sarcomeric protein (C0-C10) integral to cardiac muscle regulation. In vitro studies have demonstrated potential functional roles for regions beyond the N-terminus. However, the in vivo contributions of these domains are mostly unknown. Therefore, we examined the in vivo consequences of expression of N-terminal truncated cMyBPC (C3C10). Neonatal cMyBPC-/- mice were injected with AAV9-full length (FL), C3C10 cMyBPC, or saline, and echocardiography was performed 6 wk after injection. We then isolated skinned myocardium from virus-treated hearts and performed mechanical experiments. Our results show that expression of C3C10 cMyBPC in cMyBPC-/- mice resulted in a 28% increase in systolic ejection fraction compared to saline-injected cMyBPC-/- mice and a 25% decrease in left ventricle mass-to-body weight ratio. However, unlike expression of FL cMyBPC, there was no prolongation of ejection time compared to saline-injected mice. In vitro mechanical experiments demonstrated that functional improvements in cMyBPC-/- mice expressing C3C10 were primarily due to a 35% reduction in the rate of cross-bridge recruitment at submaximal Ca2+ concentrations when compared to hearts from saline-injected cMyBPC-/- mice. However, unlike the expression of FL cMyBPC, there was no change in the rate of cross-bridge detachment when compared to saline-injected mice. Our data demonstrate that regions of cMyBPC beyond the N-terminus are important for in vivo cardiac function, and have divergent effects on cross-bridge behavior. Elucidating the molecular mechanisms of cMyBPC region-specific function could allow for development of targeted approaches to manipulate specific aspects of cardiac contractile function.
© 2023 Dominic et al.
Figures





References
-
- Alfares, A.A., Kelly, M.A., McDermott, G., Funke, B.H., Lebo, M.S., Baxter, S.B., Shen, J., McLaughlin, H.M., Clark, E.H., Babb, L.J., et al. . 2015. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genet. med. 17:880–888. 10.1038/gim.2014.205 - DOI - PubMed
-
- Doh, C.Y., Bharambe N., Holmes J.B., Dominic K.L., Swanberg C.E., Mamidi R., Chen Y., Bandyopadhyay S., Ramachandran R., and Stelzer J.E.. 2022a. Molecular characterization of linker and loop-mediated structural modulation and hinge motion in the C4-C5 domains of cMyBPC. J. Struct. Biol. 214:107856. 10.1016/j.jsb.2022.107856 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

