New therapies for spinal muscular atrophy: where we stand and what is next
- PMID: 37067602
- PMCID: PMC10354145
- DOI: 10.1007/s00431-023-04883-8
New therapies for spinal muscular atrophy: where we stand and what is next
Abstract
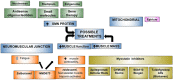
The natural history of spinal muscular atrophy has been radically changed by the advent of improved standards of care and the availability of disease-modifying therapies. The aim of this paper is to provide the current therapeutic scenario including new perspectives and to report the challenges related to new phenotypes a few years after the therapies have become available. The paper also includes a review of real-world data that provides information on safety and efficacy in individuals that were not included in clinical trials. Special attention is paid to future perspectives both in terms of new drugs that are currently investigated in clinical trials or providing details on current developments in the use of the available drugs, including combination therapies or new modalities of dose or administration. Conclusion: Clinical trials and real world data support the efficacy and safety profiles of the available drugs. At the moment there is not enough published evidence about the superiority of one product compared to the others. What is Known: • Safety and efficacy results of clinical trials have led in the last 6 years to the marketing of three drugs for spinal muscular atrophy, with different mechanisms of action. What is New: • Since the drug's approval, real-world data allow us to have data on bigger and heterogeneous groups of patients in contrast with those included in clinical trials. • In addition to the new molecules, combinations of therapies are currently being evaluated.
Keywords: Clinical trial; Combined therapies; Real-world data; Spinal muscular atrophy; Therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical