Effectiveness and Safety of Recombinant Human Follicle-Stimulating Hormone (Follitrope™) in Inducing Controlled Ovarian Stimulation in Infertile Women in Real-World Practice: a Prospective Cohort Study
- PMID: 37067727
- PMCID: PMC10480279
- DOI: 10.1007/s43032-023-01228-6
Effectiveness and Safety of Recombinant Human Follicle-Stimulating Hormone (Follitrope™) in Inducing Controlled Ovarian Stimulation in Infertile Women in Real-World Practice: a Prospective Cohort Study
Abstract
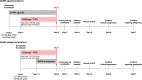
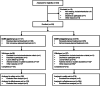
To evaluate the safety and effectiveness of recombinant human follicle-stimulating hormone (rhFSH [Follitrope™]) in infertile women undergoing in vitro fertilization (IVF). To identify predictors of ovarian response that induce optimal clinical outcomes. This multicenter prospective study enrolled infertile women who were scheduled to undergo IVF after ovarian stimulation with rhFSH (Follitrope™) following the gonadotropin-releasing hormone (GnRH) agonist or GnRH antagonist protocol. Predictive factors for ovarian response were identified in the GnRH antagonist group based on the number of oocytes retrieved. A total of 516 infertile women were enrolled, among whom 136 (except one who withdrew before administration) received rhFSH using the GnRH agonist protocol and 379 using the antagonist protocol. The mean number of oocytes retrieved was 13.4 in the GnRH agonist group and 13.6 in the GnRH antagonist group. The clinical pregnancy rates were 32.3% (30/93) and 39.9% (115/288) in the GnRH agonist and antagonist groups, respectively. The incidence of ovarian hyperstimulation syndrome was 1.8% and 3.4% in the GnRH agonist and antagonist groups, respectively. No other significant safety risks associated with rhFSH administration were identified. Body mass index, basal serum FSH and anti-Müllerian hormone levels, and antral follicle count were identified as predictors of ovarian response by multiple regression with backward elimination, and the final regression model accounted for 26.5% of the response variability. In real-world practice, rhFSH (Follitrope™) is safe and effective in inducing ovarian stimulation in infertile women. Patient characteristics identified as predictors can be considered to be highly related to optimal clinical outcomes.
Keywords: Controlled ovarian stimulation; In vitro fertilization; Ovarian response; Pregnancy; Recombinant human follicle-stimulating hormone.
© 2023. The Author(s).
Conflict of interest statement
Heemin Gwak is an employee of LG Chem, Ltd. The other authors declare that they have no conflict of interest.
Figures

References
-
- Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J, et al. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI) Cochrane Database Syst Rev. 2018;2:CD012693. - PMC - PubMed
-
- Sydow P, Gmeinwieser N, Pribbernow K, Keck C, Wiegratz I. Effectiveness and safety of follitropin alfa (Ovaleap®) for ovarian stimulation using a GnRH antagonist protocol in real-world clinical practice: a multicenter, prospective, open, non-interventional assisted reproductive technology study. Reprod Biol Endocrinol. 2020;18(1):54. doi: 10.1186/s12958-020-00610-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

