Introduction to Pharmaceutical Co-amorphous Systems Using a Green Co-milling Technique
- PMID: 37067885
- PMCID: PMC10100544
- DOI: 10.1021/acs.jchemed.3c00036
Introduction to Pharmaceutical Co-amorphous Systems Using a Green Co-milling Technique
Abstract
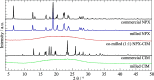
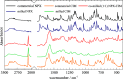
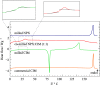
The concept of co-amorphous systems is introduced in an integrated laboratory experiment, designed for advanced chemistry students, using solvent-free, environmentally friendly mechanochemistry. The dual-drug naproxen-cimetidine co-amorphous system (NPX-CIM) is investigated as an example of the emergent field of medicinal mechanochemistry. Students are trained in solid-state characterization techniques including X-ray powder diffraction, Fourier-transform infrared spectroscopy, and thermal analysis by differential scanning calorimetry. This lab experiment also provides an opportunity to discuss the relevance of different solid forms of pharmaceutics, emphasizing particular properties of disordered materials. This experiment can easily fit the curriculum of any Chemistry or Pharmacy master level degree in courses dealing with instrumental analysis, solid state chemistry, or green chemistry, for classes of 6 to 18 students, in a 5-h lab session. Suggestions to adapt it to the use of a single characterization technique are provided.
© 2023 The Authors. Published by American Chemical Society and Division of Chemical Education, Inc.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
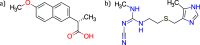
References
LinkOut - more resources
Full Text Sources
Miscellaneous
