Human herpesvirus 6-specific T-cell immunity in allogeneic hematopoietic stem cell transplant recipients
- PMID: 37067947
- PMCID: PMC10515312
- DOI: 10.1182/bloodadvances.2022009274
Human herpesvirus 6-specific T-cell immunity in allogeneic hematopoietic stem cell transplant recipients
Abstract
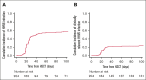
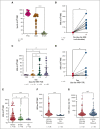
Human herpesvirus 6 (HHV-6) can reactivate after allogeneic hematopoietic stem cell transplant (allo-HSCT) and may lead to severe symptoms. HHV-6-specific immune responses after HSCT are largely unexplored. We conducted a prospective observational study on 208 consecutive adult patients who received allo-HSCT to investigate HHV-6 reactivations and specific immune responses. Interferon gamma-producing HHV-6-specific T cells were quantified using enzyme-linked immunospot assay (ELISpot). HHV-6 reactivation occurred in 63% of patients, at a median of 25 days from allo-HSCT. Only 40% of these presented a clinically relevant infection, defined by the presence of classical HHV-6 end-organ diseases (EODs), based on European Conference on Infections in Leukaemia (ECIL) guidelines, and other possible HHV6-related EODs. Using multivariate analysis, we identified risk factors for HHV-6 reactivation: previous allo-HSCT, posttransplant cyclophosphamide (PT-Cy), and time-dependent steroids introduction. The use of PT-Cy and steroids were associated with clinically relevant infections, whereas higher CD3+ cell counts seemed to be protective. Interestingly, circulating HHV-6-specific T cells were significantly higher in patients with reactivated virus. Moreover, HHV-6-specific T-cell responses, quantified at >4 days after the first viremia detection, predicted clinically relevant infections (P < .0001), with higher specificity (93%) and sensitivity (79%) than polyclonal CD3+ cells per μL. Overall survival and transplant-related mortality were not affected by time-dependent HHV-6 reactivation, whereas a significant association was observed between clinically relevant infections and acute graft-versus-host disease. These results shed light on the role of HHV-6 in allo-HSCT and may affect HHV-6 monitoring and treatment.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Styczynski J. Management of herpesvirus infections in hematopoietic cell transplant recipients. Transplantology. 2021;2(1):8–21.
-
- Oltolini C, Greco R, Galli L, et al. Infections after allogenic transplant with post-transplant cyclophosphamide: impact of donor HLA matching. Biol Blood Marrow Transplant. 2020;26(6):1179–1188. - PubMed

