Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma
- PMID: 37067952
- PMCID: PMC10463194
- DOI: 10.1182/bloodadvances.2022009260
Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma
Abstract
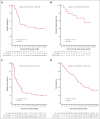
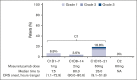
As part of a phase 1 or 2 study, this single-arm expansion cohort established the efficacy and safety of mosunetuzumab monotherapy in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) (received ≥2 previous lines of therapy). Intravenous mosunetuzumab was administered with cycle (C) 1 step-up dosing for cytokine release syndrome (CRS) mitigation: C1 day (D) 1: 1 mg; C1D8 2 mg; C1D15 and C2D1: 60 mg; C3 + D1: 30 mg. Hospitalization was not mandatory. Patients with complete response (CR) completed treatment after C8; those with partial response or stable disease continued treatment for a total of 17 cycles. The primary end point was CR rate (best response), assessed against a historical control CR rate (20%) by independent review facility. Eighty-eight patients (73.9% de novo DLBCL; 26.1% transformed follicular lymphoma) were enrolled; all had received previous anthracycline and anti-CD20 therapy. Overall response and CR rates were 42.0% (95% confidence interval [CI], 31.6-53.1) and 23.9% (95% CI, 15.4-34.1), respectively; CR rate did not reach statistical significance vs the historical control (P = .36). Median time to first response was 1.4 months. Median progression-free survival was 3.2 months (95% CI, 2.2-5.3). The CR rate in 26 patients who received previous chimeric antigen receptor T-cell (CAR-T) therapy was 12%. CRS was one of the most common adverse events (26.1% of patients); predominantly grade 1 to 2 and primarily in C1. Four patients (4.5%) discontinued mosunetuzumab owing to adverse events. Mosunetuzumab demonstrated notable efficacy and a manageable safety profile in patients with R/R DLBCL, including those previously treated with CAR-Ts. This trial was registered at www.clinicaltrials.gov as #NCT02500407.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: N.L.B. reports employment with Washington University School of Medicine; research funding from ADC Therapeutics, Autolus, Bristol Myers Squibb (BMS), Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, and Seattle Genetics; and membership on an entity’s Board of Directors or advisory committees with ADC Therapeutics, F. Hoffmann-La Roche Ltd/Genentech, Inc., and Seattle Genetics. S.A. reports a consulting role with and honoraria from Genentech, Inc./F. Hoffmann-La Roche Ltd, AstraZeneca, Novartis, BMS, Jazz, Gilead, Amgen, BeiGene, AbbVie, and Paladin; and research funding from Novartis. P.G. reports employment with Royal Adelaide Hospital; honoraria from an education session organized by F. Hoffmann-La Roche Ltd in 2021; and membership on a F. Hoffmann-La Roche Ltd Advisory Committee in 2019. S.J.S. reports a consulting or advisory role with Celgene, Nordic Nanovector, Novartis, and Pfizer; honoraria from AbbVie, Acerta, Alimera Sciences, BeiGene, AstraZeneca, Celgene, Juno Therapeutics, Genentech, Inc./F. Hoffmann-La Roche Ltd, Loxo Oncology, Nordic Nanovector, Novartis, Pfizer, and Tessa Therapeutics; research funding from AbbVie, Acerta, Adaptive Biotechnologies, Celgene/Juno, DTRM, Genentech, Inc./F. Hoffmann-La Roche Ltd, Gilead, Incyte, Merck, Novartis, Pharmacyclics, and TG Therapeutics; and a patent for combination therapies of CAR-T and PD-1 inhibitors with royalties to Novartis. C.Y.C. reports a consulting and/or advisory role and honoraria from F. Hoffmann-La Roche Ltd, Janssen, MSD, Gilead, AstraZeneca, Lilly, TG Therapeutics, BeiGene, Novartis, and BMS and research funding from BMS, F. Hoffmann-La Roche Ltd, AbbVie, and MSD. M.M. reports employment with Memorial Sloan Kettering Cancer Centre; a consulting role with ADC Therapeutics, AstraZeneca, Bayer, Daiichi Sankyo, Epizyme, F. Hoffmann-La Roche Ltd, Genentech, Inc., IMV Therapeutics, Juno Therapeutics, Karyopharm, Merck, MEI Pharma, Rocket Medical, Seattle Genetics, TG Therapeutics, Teva, and Bayer; and holds stocks in Merck. G.P.G. reports a consulting and/or advisory role and honoraria from F. Hoffmann-La Roche Ltd, Janssen, MSD, Gilead, Novartis, AstraZeneca and LINK Clinigen and research funding from MSD, BeiGene, Janssen and AbbVie. D.H.Y. reports a consulting role with GI cell, AB clone, and Pharos iBio; research funding from Samyang, Boryung, BeiGene, Sanofi, Celltrion, and Janssen; and honoraria from F. Hoffmann-La Roche Ltd, Janssen, Amgen, BMS, Novartis, Celltrion, Samyang, Boryung, Kirin Pharm, Takeda, GlaxoSmithKline, and Janssen. M.S. reports a consulting and/or advisory role with AbbVie, Genentech, Inc., AstraZeneca, Sound Biologics, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate Therapeutics, MEI Pharma, and Atara Biotherapeutic; and research funding from Mustang Bio, Celgene, BMS, Pharmacyclics, Gilead, Genentech, Inc., AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, MorphoSys/Incyte, and Vincrex. K.F. reports employment with St. Vincent’s Hospital, Sydney, Australia. S.-S.Y. reports a consulting or advisory role with Amgen, Antengene, Astellas, Celgene, Janssen, Novartis, and Takeda; honoraria from Novartis; and research funding from Kyowa Kirin, F. Hoffmann-La Roche Ltd/Genentech, Inc., and Yuhan Pharmaceutical. C.P. reports a consulting or advisory role with F. Hoffmann-La Roche Ltd, Janssen, Gilead, Incyte, and BeiGene. I.F. reports a consulting role with AbbVie, AstraZeneca, BeiGene, Century Therapeutics, Genentech, Inc., Genmab, Gilead Sciences, Great Point Partners, Hutchison MediPharma, Iksuda Therapeutics, InnoCare Pharma, Janssen, Juno Therapeutics, Kite Pharma, MorphoSys, Novartis, Nurix Therapeutics, Pharmacyclics, F. Hoffmann-La Roche Ltd, Seattle Genetics, Servier Pharmaceuticals, Takeda, TG Therapeutics, Unum Therapeutics, Verastem, Vincerx Pharma, and Yingli Pharmaceuticals; and research funding from AbbVie, Acerta Pharma, Agios, ArQule, AstraZeneca, BeiGene, Biopath, BMS, CALIBR, CALGB, Celgene, City of Hope National Medical Center, Constellation Pharmaceuticals, Curis, CTI Biopharma, Fate Therapeutics, Forma Therapeutics, Forty Seven, Genentech, Inc., Gilead Sciences, InnoCare Pharma, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Janssen, Kite Pharma, Loxo, Merck, Millennium Pharmaceuticals, MorphoSys, Myeloid Therapeutics, Novartis, Nurix, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, F. Hoffmann-La Roche Ltd, Seattle Genetics, Tessa Therapeutics, TCR2 Therapeutics, TG Therapeutics, Trillium Therapeutics, Triphase Research & Development Corp, Unum Therapeutics, and Verastem (payments made to Sarah Cannon Research Institute for all consulting and research funding). A.J. reports a consulting or advisory role with F. Hoffmann-La Roche Ltd, BeiGene, Novartis, and Sanofi. F.B. reports employment with Vall D’Hebron University Hospital, Barcelona; a consulting role with F. Hoffmann-La Roche Ltd, Genentech, Inc., Novartis, Janssen, AbbVie, Gilead/Kite, Mundipharma, Takeda, Celgene/BMS, AstraZeneca, Lilly, BeiGene, TG Therapeutics, Advantage Allogene, Lava Therapeutics, and Enterome; research funding from F. Hoffmann-La Roche Ltd, Genentech, Inc., AbbVie, Janssen, Lilly, AstraZeneca, Novartis, Kite, BMS, Takeda, TG Therapeutics, BeiGene, Advantage, Allogene, Lava Therapeutics, and Enterome; honoraria from F. Hoffmann-La Roche Ltd, Genentech, Inc., Novartis, Janssen, AbbVie, Gilead/Kite, Mundipharma, Takeda, Celgene/BMS, AstraZeneca, Lilly, BeiGene, TG Therapeutics, Advantage, Allogene, Lava Therapeutics, and Enterome; speaker's bureaus with F. Hoffmann-La Roche Ltd, Genentech, Inc., Novartis, Janssen, AbbVie, Gilead/Kite, Mundipharma, Takeda, Celgene/BMS, AstraZeneca, Lilly, BeiGene, TG Therapeutics, Advantage, Allogene, Lava Therapeutics, and Enterome; and other from F. Hoffmann-La Roche Ltd, Novartis, Janssen, AbbVie, Gilead, Mundipharma, Celgene/BMS, Takeda, and AstraZeneca. M.C.W. and S.Y. report employment with Genentech, Inc.; meeting attendance and/or travel support from F. Hoffmann-La Roche Ltd/Genentech, Inc.; and stocks and stock options in F. Hoffmann-La Roche Ltd. I.T. and E.P. report employment with and are equity holders in Genentech, Inc. C.-C.L. reports employment with and stocks and stock options in F. Hoffmann-La Roche Ltd, and a patent for dosing for treatment with anti-CD20/anti-CD3 bispecific antibodies. H.H. reports employment with F. Hoffmann-La Roche Ltd. A.K. reports employment with and holds stocks in F. Hoffmann-La Roche Ltd. L.E.B. reports consulting fees from Genentech, Inc., ADC Therapeutics, Merck, AstraZeneca and Amgen, and participation on a Data Safety Monitoring Board for Ziopharm Oncology. L.H.S. declares no competing financial interests.
The current affiliation for M.M. is Rutgers Cancer Institute of New Jersey, New Brunswick, NJ.
Figures

References
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32. - PubMed
-
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116–v125. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

