Ancestral protein reconstruction reveals evolutionary events governing variation in Dicer helicase function
- PMID: 37068011
- PMCID: PMC10159624
- DOI: 10.7554/eLife.85120
Ancestral protein reconstruction reveals evolutionary events governing variation in Dicer helicase function
Abstract
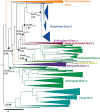
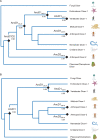
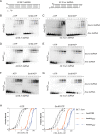
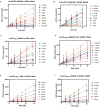
Antiviral defense in ecdysozoan invertebrates requires Dicer with a helicase domain capable of ATP hydrolysis. But despite well-conserved ATPase motifs, human Dicer is incapable of ATP hydrolysis, consistent with a muted role in antiviral defense. To investigate this enigma, we used ancestral protein reconstruction to resurrect Dicer's helicase in animals and trace the evolutionary trajectory of ATP hydrolysis. Biochemical assays indicated ancient Dicer possessed ATPase function, that like extant invertebrate Dicers, is stimulated by dsRNA. Analyses revealed that dsRNA stimulates ATPase activity by increasing ATP affinity, reflected in Michaelis constants. Deuterostome Dicer-1 ancestor, while exhibiting lower dsRNA affinity, retained some ATPase activity; importantly, ATPase activity was undetectable in the vertebrate Dicer-1 ancestor, which had even lower dsRNA affinity. Reverting residues in the ATP hydrolysis pocket was insufficient to rescue hydrolysis, but additional substitutions distant from the pocket rescued vertebrate Dicer-1's ATPase function. Our work suggests Dicer lost ATPase function in the vertebrate ancestor due to loss of ATP affinity, involving motifs distant from the active site, important for coupling dsRNA binding to the active conformation. By competing with Dicer for viral dsRNA, RIG-I-like receptors important for interferon signaling may have allowed or actively caused loss of ATPase function.
Keywords: RIG-I; antiviral defense; biochemistry; chemical biology; dsRNA; evolutionary biology; innate immunity; none; phylogenetic; ribonuclease III.
© 2023, Aderounmu et al.
Conflict of interest statement
AA, PA, BK, BB No competing interests declared
Figures









Update of
- doi: 10.1101/2022.12.30.522297
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

