Digital and Absolute Assays for Low Abundance Molecular Biomarkers
- PMID: 37068158
- PMCID: PMC10163903
- DOI: 10.1021/acs.accounts.3c00030
Digital and Absolute Assays for Low Abundance Molecular Biomarkers
Abstract
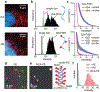
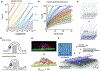
There has been a recent surge of advances in biomolecular assays based on the measurement of discrete molecular targets as opposed to signals averaged across molecular ensembles. Many of these "digital" assay designs derive from now-mature technologies involving single-molecule imaging and microfluidics and provide an assortment of new modalities to quantify nucleic acids and proteins in biospecimens such as blood and tissue homogenates. A primary new benefit is the robust detection of trace analytes at attomolar to femtomolar concentrations for which many ensemble assays cannot distinguish signals above noise levels. In addition, multiple biomolecules can be differentiated within a mixture using optical barcodes, with much faster and simpler readouts compared with sequencing methods. In ideal digital assays, signals should, in theory, further represent absolute molecular counts, rather than relative levels, eliminating the need for calibration standards that are the mainstay of typical assays. Several digital assay platforms have now been commercialized but challenges hinder the adoption and diversification of these new formats, as there are broad needs to balance sensitivity and dynamic range of detection, increase analyte multiplexing, improve sample throughput, and reduce cost. Our lab and others have developed technologies to address these challenges by redesigning molecular probes and labels, improving molecular transport within detection focal volumes, and applying solution-based readout methods in flow.This Account describes the principles, formats, and design constraints of digital biomolecular assays that apply optical labels toward the goal of simple and routine target counting that may ultimately approach absolute readout standards. The primary challenges can be understood from fundamental concepts in thermodynamics and kinetics of association reactions, mass transport, and discrete statistics. Major advances include (1) new inorganic nanocrystal probes for more robust counting compared with dyes, (2) diverse molecular amplification tools that endow attachment of numerous labels to single targets, (3) specialized surfaces with patterned features for electromagnetic coupling to labels for signal amplification, (4) surface capture enhancement methods to concentrate targets through disruption of diffusion depletion zones, and (5) flow counting in which analytes are rapidly counted in solution without pull-down to a surface. Further progress and integration of these tools for biomolecular counting could improve the precision of laboratory measurements in life sciences research and benefit clinical diagnostic assays for low abundance biomarkers in limiting biospecimen volumes that are out of reach of traditional ensemble-level bioassays.
Figures

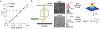
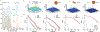


Similar articles
-
Ultrasensitive electrochemical biomolecular detection using nanostructured microelectrodes.Acc Chem Res. 2014 Aug 19;47(8):2417-25. doi: 10.1021/ar500130m. Epub 2014 Jun 25. Acc Chem Res. 2014. PMID: 24961296
-
High-Throughput, High-Multiplex Digital Protein Detection with Attomolar Sensitivity.ACS Nano. 2022 Jan 25;16(1):1025-1035. doi: 10.1021/acsnano.1c08675. Epub 2022 Jan 14. ACS Nano. 2022. PMID: 35029381 Free PMC article.
-
Fluorescent Biosensors Based on Single-Molecule Counting.Acc Chem Res. 2016 Sep 20;49(9):1722-30. doi: 10.1021/acs.accounts.6b00237. Epub 2016 Sep 1. Acc Chem Res. 2016. PMID: 27583695 Review.
-
Direct Kinetic Fingerprinting for High-Accuracy Single-Molecule Counting of Diverse Disease Biomarkers.Acc Chem Res. 2021 Jan 19;54(2):388-402. doi: 10.1021/acs.accounts.0c00621. Epub 2020 Dec 31. Acc Chem Res. 2021. PMID: 33382587 Free PMC article.
-
Advances in Optical Single-Molecule Detection: En Route to Supersensitive Bioaffinity Assays.Angew Chem Int Ed Engl. 2020 Jun 26;59(27):10746-10773. doi: 10.1002/anie.201913924. Epub 2020 Apr 15. Angew Chem Int Ed Engl. 2020. PMID: 31869502 Free PMC article. Review.
Cited by
-
Pushing the Limits of Lateral Flow Immunoassay by Digital SERS for the Ultralow Detection of SARS-CoV-2 Virus.Small Sci. 2024 Aug 10;4(11):2400259. doi: 10.1002/smsc.202400259. eCollection 2024 Nov. Small Sci. 2024. PMID: 40213455 Free PMC article.
-
Ultrasensitive Chemical Analysis on Gold Nano Popcorn Substrate Using Digital Surface-Enhanced Raman Scattering.Molecules. 2025 Mar 19;30(6):1371. doi: 10.3390/molecules30061371. Molecules. 2025. PMID: 40142146 Free PMC article.
-
Nanoarchitectonics-based electrochemical aptasensors for highly efficient exosome detection.Sci Technol Adv Mater. 2024 Apr 22;25(1):2345041. doi: 10.1080/14686996.2024.2345041. eCollection 2024. Sci Technol Adv Mater. 2024. PMID: 38742153 Free PMC article. Review.
References
-
-
Smith L; Kohli M; Smith AM Expanding the Dynamic Range of Fluorescence Assays through Single-Molecule Counting and Intensity Calibration. J. Am. Chem. Soc 2018, 140 (42), 13904–13912.
Continuity of dynamic range across digital and ensemble fluorescence assays is achieved by calibrating single molecule intensities on an imaging substrate.
-
-
-
Le P; Lim SJ; Baculis BC; Chung HJ; Kilian KA; Smith AM Counting Growth Factors in Single Cells with Infrared Quantum Dots to Measure Discrete Stimulation Distributions. Nat. Commun 2019, 10 (1), 909.
Absolute counting of labeled proteins is achieved in single cells through three-dimensional imaging of individual quantum dots.
-
-
-
Smith LD; Liu Y; Zahid MU; Canady TD; Wang L; Kohli M; Cunningham BT; Smith AM High-Fidelity Single Molecule Quantification in a Flow Cytometer Using Multiparametric Optical Analysis. ACS Nano 2020, 14 (2), 2324–2335.
Flow counting is used to quantify nucleic acids that are enzymatically extended and labeled with dyes without purification.
-
-
-
Xiong Y; Huang Q; Canady TD; Barya P; Liu S; Arogundade OH; Race CM; Che C; Wang X; Zhou L; Wang X; Kohli M; Smith AM; Cunningham BT Photonic Crystal Enhanced Fluorescence Emission and Blinking Suppression for Single Quantum Dot Digital Resolution Biosensing. Nat. Commun 2022, 13 (1), 4647.
Photonic crystal surfaces improve the sensitivity of digital surface capture assays to allow the use of low numerical aperture objectives and inexpensive optical setups.
-
-
- Lequin RM Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem 2005, 51 (12), 2415–2418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

