The human malaria parasite Plasmodium falciparum can sense environmental changes and respond by antigenic switching
- PMID: 37068249
- PMCID: PMC10151525
- DOI: 10.1073/pnas.2302152120
The human malaria parasite Plasmodium falciparum can sense environmental changes and respond by antigenic switching
Abstract
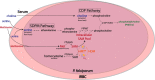
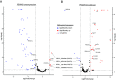
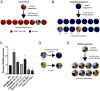
The primary antigenic and virulence determinant of the human malaria parasite Plasmodium falciparum is a variant surface protein called PfEMP1. Different forms of PfEMP1 are encoded by a multicopy gene family called var, and switching between active genes enables the parasites to evade the antibody response of their human hosts. var gene switching is key for the maintenance of chronic infections; however, what controls switching is unknown, although it has been suggested to occur at a constant frequency with little or no environmental influence. var gene transcription is controlled epigenetically through the activity of histone methyltransferases (HMTs). Studies in model systems have shown that metabolism and epigenetic control of gene expression are linked through the availability of intracellular S-adenosylmethionine (SAM), the principal methyl donor in biological methylation modifications, which can fluctuate based on nutrient availability. To determine whether environmental conditions and changes in metabolism can influence var gene expression, P. falciparum was cultured in media with altered concentrations of nutrients involved in SAM metabolism. We found that conditions that influence lipid metabolism induce var gene switching, indicating that parasites can respond to changes in their environment by altering var gene expression patterns. Genetic modifications that directly modified expression of the enzymes that control SAM levels similarly led to profound changes in var gene expression, confirming that changes in SAM availability modulate var gene switching. These observations directly challenge the paradigm that antigenic variation in P. falciparum follows an intrinsic, programed switching rate, which operates independently of any external stimuli.
Keywords: gene expression; immune evasion; metabolism; methylation; var genes.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Boddey J. A., Cowman A. F., Plasmodium nesting: Remaking the erythrocyte from the inside out. Annu. Rev. Microbiol. 67, 243–269 (2013). - PubMed
-
- Su X., et al. , A large and diverse gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–100 (1995). - PubMed
-
- Baruch D. I., et al. , Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

