Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee
- PMID: 37068329
- PMCID: PMC10192339
- DOI: 10.1016/j.clinph.2023.03.010
Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee
Abstract
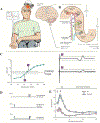
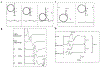
The review provides a comprehensive update (previous report: Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2008;119(3):504-32) on clinical diagnostic utility of transcranial magnetic stimulation (TMS) in neurological diseases. Most TMS measures rely on stimulation of motor cortex and recording of motor evoked potentials. Paired-pulse TMS techniques, incorporating conventional amplitude-based and threshold tracking, have established clinical utility in neurodegenerative, movement, episodic (epilepsy, migraines), chronic pain and functional diseases. Cortical hyperexcitability has emerged as a diagnostic aid in amyotrophic lateral sclerosis. Single-pulse TMS measures are of utility in stroke, and myelopathy even in the absence of radiological changes. Short-latency afferent inhibition, related to central cholinergic transmission, is reduced in Alzheimer's disease. The triple stimulation technique (TST) may enhance diagnostic utility of conventional TMS measures to detect upper motor neuron involvement. The recording of motor evoked potentials can be used to perform functional mapping of the motor cortex or in preoperative assessment of eloquent brain regions before surgical resection of brain tumors. TMS exhibits utility in assessing lumbosacral/cervical nerve root function, especially in demyelinating neuropathies, and may be of utility in localizing the site of facial nerve palsies. TMS measures also have high sensitivity in detecting subclinical corticospinal lesions in multiple sclerosis. Abnormalities in central motor conduction time or TST correlate with motor impairment and disability in MS. Cerebellar stimulation may detect lesions in the cerebellum or cerebello-dentato-thalamo-motor cortical pathways. Combining TMS with electroencephalography, provides a novel method to measure parameters altered in neurological disorders, including cortical excitability, effective connectivity, and response complexity.
Keywords: Motor evoked potential; Neurological disorders; Short interval intracortical inhibition; Transcranial magnetic stimulation.
Copyright © 2023 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
Figures



References
-
- Abbruzzese G, Buccolieri A, Marchese R, Trompetto C, Mandich P, Schieppati M. Intracortical inhibition and facilitation are abnormal in Huntington’s disease: a paired magnetic stimulation study. Neurosci Lett 1997;228(2):87–90. - PubMed
-
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain 2001;124:537–45. - PubMed
-
- Abbruzzese G, Tabaton M, Morena M, Dall’Agata D, Favale E. Motor and sensory evoked potentials in progressive supranuclear palsy. Mov Disord 1991;6(1):49–54. - PubMed
-
- Afra J, Mascia A, Gérard P, Maertens de Noordhout A, Schoenen J. Interictal cortical excitability in migraine: a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol 1998;44(2):209–15. - PubMed

