Integrating tuberculosis and COVID-19 molecular testing in Lima, Peru: a cross-sectional, diagnostic accuracy study
- PMID: 37068500
- PMCID: PMC10105319
- DOI: 10.1016/S2666-5247(23)00042-3
Integrating tuberculosis and COVID-19 molecular testing in Lima, Peru: a cross-sectional, diagnostic accuracy study
Abstract
Background: Integrated molecular testing could be an opportunity to detect and provide care for both tuberculosis and COVID-19. Many high tuberculosis burden countries, such as Peru, have existing GeneXpert systems for tuberculosis testing with GeneXpert Xpert MTB/RIF Ultra (Xpert Ultra), and a GeneXpert SARS-CoV-2 assay, GeneXpert Xpert Xpress SARS-CoV-2 (Xpert Xpress), is also available. We aimed to assess the feasibility of integrating tuberculosis and COVID-19 testing using one sputum specimen with Xpert Ultra and Xpert Xpress in Lima, Peru.
Methods: In this cross-sectional, diagnostic accuracy study, we recruited adults presenting with clinical symptoms or suggestive history of tuberculosis or COVID-19, or both. Participants were recruited from a total of 35 primary health facilities in Lima, Peru. Participants provided one nasopharyngeal swab and one sputum sample. For COVID-19, we tested nasopharyngeal swabs and sputum using Xpert Xpress; for tuberculosis, we tested sputum using culture and Xpert Ultra. We compared diagnostic accuracy of sputum testing using Xpert Xpress with nasopharyngeal swab testing using Xpert Xpress. Individuals with positive Xpert Xpress nasopharyngeal swab results were considered COVID-19 positive, and a positive culture indicated tuberculosis. To assess testing integration, the proportion of cases identified in sputum by Xpert Xpress was compared with Xpert Xpress on nasopharyngeal swabs, and sputum by Xpert Ultra was compared with culture.
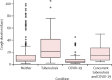
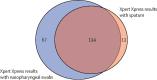
Findings: Between Jan 11, 2021, and April 26, 2022, we recruited 600 participants (312 [52%] women and 288 [48%] men). In-study prevalence of tuberculosis was 13% (80 participants, 95% CI 11-16) and of SARS-CoV-2 was 35% (212 participants, 32-39). Among tuberculosis cases, 13 (2·2%, 1·2-3·7) participants were concurrently positive for SARS-CoV-2. Regarding the diagnostic yield of integrated testing, Xpert Ultra detected 96% (89-99) of culture-confirmed tuberculosis cases (n=77), and Xpert Xpress-sputum detected 67% (60-73) of COVID-19 cases (n=134). All five study staff reported that integrated molecular testing was easy and acceptable.
Interpretation: The diagnostic yield of Xpert Xpress on sputum was moderate, but integrated testing for tuberculosis and COVID-19 with GeneXpert was feasible. However, systematic testing for both diseases might not be the ideal approach for everyone presenting with presumptive tuberculosis or COVID-19, as concurrent positive cases were rare during the study period. Further research might help to identify when integrated testing is most worthwhile and its optimal implementation.
Funding: Canadian Institutes of Health Research and International Development Research Centre.
Translation: For the Spanish translation of the abstract see Supplementary Materials section.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests MP serves as an advisor to the following non-profit agencies in global health: Bill & Melinda Gates Foundation, Foundation for Innovative New Diagnostics, WHO, and Stop TB Partnership. CU-G reports receiving funding from the Foundation of Innovative New Diagnostics, US National Institutes of Health, Abbott, and National Research Council Canada for grants in tuberculosis or COVID research, or both; receiving payment or honoraria for lectures, presentations, or educational events from Abbott and Molbio; and participating on an advisory board for point-of-care testing in infectious diseases.
Figures
Similar articles
-
Tuberculosis/COVID-19 co-infection detected in a single sputum sample using a rapid molecular test.Braz J Microbiol. 2022 Jun;53(2):633-639. doi: 10.1007/s42770-021-00677-y. Epub 2022 Feb 2. Braz J Microbiol. 2022. PMID: 35107805 Free PMC article.
-
Optimal processing of tongue swab samples for Mycobacterium tuberculosis detection by the Xpert MTB/RIF Ultra assay.Microbiol Spectr. 2025 Mar 4;13(3):e0240324. doi: 10.1128/spectrum.02403-24. Epub 2025 Jan 28. Microbiol Spectr. 2025. PMID: 39873526 Free PMC article.
-
Diagnostic accuracy of tongue swab testing on two automated tuberculosis diagnostic platforms, Cepheid Xpert MTB/RIF Ultra and Molbio Truenat MTB Ultima.J Clin Microbiol. 2024 Apr 10;62(4):e0001924. doi: 10.1128/jcm.00019-24. Epub 2024 Mar 14. J Clin Microbiol. 2024. PMID: 38483169 Free PMC article.
-
Systematic Review of Pooling Sputum as an Efficient Method for Xpert MTB/RIF Tuberculosis Testing during the COVID-19 Pandemic.Emerg Infect Dis. 2021 Mar;27(3):719-727. doi: 10.3201/eid2703.204090. Emerg Infect Dis. 2021. PMID: 33622482 Free PMC article.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
Cited by
-
Xpert MTB/RIF Ultra assay for pulmonary tuberculosis and rifampicin resistance in adults and adolescents.Cochrane Database Syst Rev. 2025 Jul 29;7(7):CD009593. doi: 10.1002/14651858.CD009593.pub6. Cochrane Database Syst Rev. 2025. PMID: 40728034 Free PMC article.
-
Prior COVID-19 infection among newly diagnosed tuberculosis patients in a tertiary care center in Tehran: A case-control study.Immun Inflamm Dis. 2024 May;12(5):e1275. doi: 10.1002/iid3.1275. Immun Inflamm Dis. 2024. PMID: 38804889 Free PMC article.
-
Performance evaluation of the Molbio diagnostics Truenat MTB Ultima/COVID-19 multiplex assay for TB and COVID-19 case detection among people with symptoms suggestive of tuberculosis-a study protocol for clinical trials.Front Public Health. 2025 Jun 27;13:1620210. doi: 10.3389/fpubh.2025.1620210. eCollection 2025. Front Public Health. 2025. PMID: 40655205 Free PMC article.
References
-
- WHO . World Health Organization; Geneva: 2022. Global tuberculosis report 2022.
-
- Global Preparedness Monitoring Board . World Health Organization; Geneva: 2020. A world in disorder: Global Preparedness Monitoring Board annual report 2020.
-
- US Agency for International Development. Stop TB Partnership . Stop TB Partnership; Geneva: 2021. Simultaneous, integrated diagnostic testing approach to detect COVID-19 and TB in high TB burden countries.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

