Trial of thromboxane receptor inhibition with ifetroban: TP receptors regulate eicosanoid homeostasis in aspirin-exacerbated respiratory disease
- PMID: 37068712
- PMCID: PMC10524565
- DOI: 10.1016/j.jaci.2023.03.030
Trial of thromboxane receptor inhibition with ifetroban: TP receptors regulate eicosanoid homeostasis in aspirin-exacerbated respiratory disease
Abstract
Background: Aspirin-exacerbated respiratory disease (AERD) is the triad of asthma, nasal polyposis, and respiratory reactions to COX-1 inhibitors. Overproduction of cysteinyl leukotrienes and underproduction of prostaglandin E2 (PGE2) are hallmarks of AERD. A mouse model predicted a key role for the thromboxane-prostanoid (TP) receptor in AERD.
Objective: Our aim was to determine whether ifetroban, a TP receptor antagonist, attenuates aspirin-induced respiratory symptoms in patients with AERD.
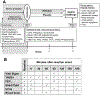
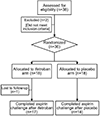
Methods: A total of 35 patients with AERD completed a 4-week double-blinded, placebo-controlled trial of ifetroban and underwent an oral aspirin challenge. The primary outcome was change in the provocative dose of aspirin that caused a 2-point increase in Total Nasal Symptom Score. Changes in lung function, eicosanoid levels, and platelet and mast cell activation were assessed. Cultured human nasal fibroblasts were stimulated with or without the TP agonist U46619 and assayed for prostanoid production.
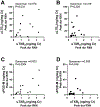
Results: Ifetroban was well tolerated in AERD and did not change the mean 2-point increase in Total Nasal Symptom Score (P = .763). Participants taking ifetroban had greater aspirin-induced nasal symptoms and a greater decline in FEV1 value than did participants receiving placebo (-18.8% ± 3.6% with ifetroban vs -8.4% ± 2.1% with placebo [P = .017]). Four weeks of ifetroban significantly increased urinary leukotriene E4 levels and decreased nasal PGE2 levels compared with placebo. Peak aspirin-induced urinary thromboxane levels correlated with peak urinary leukotriene E4 and prostaglandin D2 metabolite levels in participants taking ifetroban. U46119 significantly potentiated the production of PGE2 by cultured nasal fibroblasts from subjects with AERD but not by cultured nasal fibroblasts from controls without polypoid sinusitis.
Conclusion: Contrary to our hypothesis, TP receptor blockade worsened aspirin-induced reactions in AERD, possibly by exacerbating dysregulation of the eicosanoid system. TP signaling on stromal cells may be critical to maintaining PGE2 production when COX-2 function is low.
Keywords: AERD; Aspirin-exacerbated respiratory disease; Samter triad; ifetroban; mast cell; platelet; thromboxane receptor.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


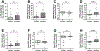

References
-
- Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135(3):676–81 e1. - PubMed
-
- Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Am Rev Respir Dis. 1993;148(6 Pt 1):1447–51. - PubMed
-
- Dahlen B, Nizankowska E, Szczeklik A, Zetterstrom O, Bochenek G, Kumlin M, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1187–94. - PubMed
-
- Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol. 2011;128(1):66–72. - PubMed
-
- Luo M, Jones SM, Flamand N, Aronoff DM, Peters-Golden M, Brock TG. Phosphorylation by protein kinase a inhibits nuclear import of 5-lipoxygenase. J Biol Chem. 2005;280(49):40609–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

