Personalised recommendations for hospitalised patients with Acute Kidney Injury using a Kidney Action Team (KAT-AKI): protocol and early data of a randomised controlled trial
- PMID: 37068906
- PMCID: PMC10111926
- DOI: 10.1136/bmjopen-2023-071968
Personalised recommendations for hospitalised patients with Acute Kidney Injury using a Kidney Action Team (KAT-AKI): protocol and early data of a randomised controlled trial
Abstract
Introduction: Although studies have examined the utility of clinical decision support tools in improving acute kidney injury (AKI) outcomes, no study has evaluated the effect of real-time, personalised AKI recommendations. This study aims to assess the impact of individualised AKI-specific recommendations delivered by trained clinicians and pharmacists immediately after AKI detection in hospitalised patients.
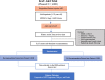
Methods and analysis: KAT-AKI is a multicentre randomised investigator-blinded trial being conducted across eight hospitals at two major US hospital systems planning to enrol 4000 patients over 3 years (between 1 November 2021 and 1 November 2024). A real-time electronic AKI alert system informs a dedicated team composed of a physician and pharmacist who independently review the chart in real time, screen for eligibility and provide combined recommendations across the following domains: diagnostics, volume, potassium, acid-base and medications. Recommendations are delivered to the primary team in the alert arm or logged for future analysis in the usual care arm. The planned primary outcome is a composite of AKI progression, dialysis and mortality within 14 days from randomisation. A key secondary outcome is the percentage of recommendations implemented by the primary team within 24 hours from randomisation. The study has enrolled 500 individuals over 8.5 months. Two-thirds were on a medical floor at the time of the alert and 17.8% were in an intensive care unit. Virtually all participants were recommended for at least one diagnostic intervention. More than half (51.6%) had recommendations to discontinue or dose-adjust a medication. The median time from AKI alert to randomisation was 28 (IQR 15.8-51.5) min.
Ethics and dissemination: The study was approved by the ethics committee of each study site (Yale University and Johns Hopkins institutional review board (IRB) and a central IRB (BRANY, Biomedical Research Alliance of New York). We are committed to open dissemination of the data through clinicaltrials.gov and sharing of data on an open repository as well as publication in a peer-reviewed journal on completion.
Trial registration number: NCT04040296.
Keywords: Acute renal failure; Adult nephrology; INTENSIVE & CRITICAL CARE; Nephrology; Quality in health care.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FPW reports research funding unrelated to this project from AstraZeneca, Boehringer-Ingelheim, Vifor Pharma and Whoop, Inc. CP is a member of the advisory board of and owns equity in RenalytixAI. He also serves as a consultant for Genfit and Novartis. All other authors have no disclosures.
Figures

Similar articles
-
Electronic Alerts for Acute Kidney Injury Amelioration (ELAIA-1): a completely electronic, multicentre, randomised controlled trial: design and rationale.BMJ Open. 2019 Jun 1;9(5):e025117. doi: 10.1136/bmjopen-2018-025117. BMJ Open. 2019. PMID: 31154298 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A randomised, double-blind, placebo-controlled, pilot trial of intravenous plasma purified alpha-1 antitrypsin for SARS-CoV-2-induced Acute Respiratory Distress Syndrome: a structured summary of a study protocol for a randomised, controlled trial.Trials. 2021 Apr 19;22(1):288. doi: 10.1186/s13063-021-05254-0. Trials. 2021. PMID: 33874981 Free PMC article.
-
Electronic Alerts for Acute Kidney Injury.Dtsch Arztebl Int. 2017 Jan 9;114(1-02):1-8. doi: 10.3238/arztebl.2017.0001. Dtsch Arztebl Int. 2017. PMID: 28143633 Free PMC article.
-
Postdischarge Care of Acute Kidney Injury Survivors: An Opportunity for Targeted Nurse and Pharmacist Interventions.Adv Kidney Dis Health. 2025 Mar;32(2):154-161. doi: 10.1053/j.akdh.2025.01.005. Adv Kidney Dis Health. 2025. PMID: 40222802 Review.
Cited by
-
Effects of inpatient creatinine testing frequency on acute kidney injury identification and staging: a historical cohort study.Int J Clin Pharm. 2024 Jun;46(3):623-630. doi: 10.1007/s11096-023-01697-4. Epub 2024 Feb 5. Int J Clin Pharm. 2024. PMID: 38315304 Free PMC article.
-
Early, Individualized Recommendations for Hospitalized Patients With Acute Kidney Injury: A Randomized Clinical Trial.JAMA. 2024 Dec 24;332(24):2081-2090. doi: 10.1001/jama.2024.22718. JAMA. 2024. PMID: 39454050 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
