Identifying women who may benefit from higher dose omega-3 supplementation during pregnancy to reduce their risk of prematurity: exploratory analyses from the ORIP trial
- PMID: 37068907
- PMCID: PMC10111924
- DOI: 10.1136/bmjopen-2022-070220
Identifying women who may benefit from higher dose omega-3 supplementation during pregnancy to reduce their risk of prematurity: exploratory analyses from the ORIP trial
Abstract
Objectives: The risk factors for prematurity are multifactorial and include low omega-3 status. Omega-3 supplementation in pregnancy has been found to reduce prematurity risk, particularly among women with low omega-3 levels. This study aimed to identify maternal characteristics that predict whether women with a singleton pregnancy will benefit from omega-3 supplementation to reduce their risk of prematurity.
Design: Exploratory analyses of a multicentre, double-blind randomised trial.
Setting: 6 tertiary care centres in four states in Australia.
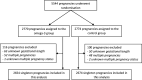
Participants: 5328 singleton pregnancies in 5305 women recruited before 20 weeks of gestation.
Interventions: Fish oil capsules containing 900 mg omega-3 long-chain polyunsaturated fatty acids per day versus vegetable oil capsules consumed from enrolment until 34 weeks' gestation.
Outcome measures: Early preterm birth (EPTB, <34 weeks' gestation) and preterm birth (PTB, <37 weeks' gestation) analysed using logistic regression models with interactions between treatment group and a range of maternal biological, clinical and demographic characteristics.
Results: Omega-3 supplementation reduced the odds of EPTB for women with low total omega-3 status in early pregnancy (OR=0.30, 95% CI 0.10-0.93). No additional maternal characteristics influenced whether omega-3 supplementation reduced the odds of EPTB. For PTB, women were more likely to benefit from omega-3 supplementation if they were multiparous (OR=0.65, 95% CI 0.49-0.87) or avoided alcohol in the lead up to pregnancy (OR=0.62, 95% CI 0.45-0.86).
Conclusions: Our results support previous findings that women with low total omega-3 levels in early pregnancy are most likely to benefit from taking omega-3 supplements to reduce their risk of EPTB. Understanding how other maternal characteristics influence the effectiveness of omega-3 supplementation on reducing PTB requires further investigation.
Trial registration number: ACTRN12613001142729.
Keywords: NEONATOLOGY; NUTRITION & DIETETICS; OBSTETRICS; PUBLIC HEALTH.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RAG has received supplies from Croda UK, prepared supplies for a trial for Efamol/Wassen UK and holds a patent (WO2013/104025A1) on stabilising and analysing fatty acids in a biological sample stored on solid media, owned by Adelaide Research and Innovation, The University of Adelaide, and licensed to Xerion. SKT, FH, SD and ISZ are employees of Société des Produits Nestlé (SPN). MM has received supplies from Croda UK and prepared supplies for a trial for Efamol/Wassen UK.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources