Promyelinating drugs promote functional recovery in an autism spectrum disorder mouse model of Pitt-Hopkins syndrome
- PMID: 37068912
- PMCID: PMC10393406
- DOI: 10.1093/brain/awad057
Promyelinating drugs promote functional recovery in an autism spectrum disorder mouse model of Pitt-Hopkins syndrome
Abstract
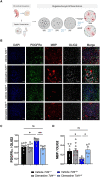
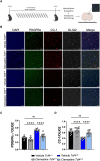
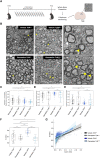
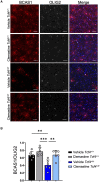
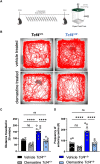
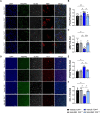
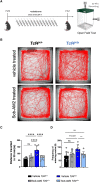
Pitt-Hopkins syndrome is an autism spectrum disorder caused by autosomal dominant mutations in the human transcription factor 4 gene (TCF4). One pathobiological process caused by murine Tcf4 mutation is a cell autonomous reduction in oligodendrocytes and myelination. In this study, we show that the promyelinating compounds, clemastine, sobetirome and Sob-AM2 are effective at restoring myelination defects in a Pitt-Hopkins syndrome mouse model. In vitro, clemastine treatment reduced excess oligodendrocyte precursor cells and normalized oligodendrocyte density. In vivo, 2-week intraperitoneal administration of clemastine also normalized oligodendrocyte precursor cell and oligodendrocyte density in the cortex of Tcf4 mutant mice and appeared to increase the number of axons undergoing myelination, as EM imaging of the corpus callosum showed a significant increase in the proportion of uncompacted myelin and an overall reduction in the g-ratio. Importantly, this treatment paradigm resulted in functional rescue by improving electrophysiology and behaviour. To confirm behavioural rescue was achieved via enhancing myelination, we show that treatment with the thyroid hormone receptor agonist sobetirome or its brain penetrating prodrug Sob-AM2, was also effective at normalizing oligodendrocyte precursor cell and oligodendrocyte densities and behaviour in the Pitt-Hopkins syndrome mouse model. Together, these results provide preclinical evidence that promyelinating therapies may be beneficial in Pitt-Hopkins syndrome and potentially other neurodevelopmental disorders characterized by dysmyelination.
Keywords: ASD; Pitt–Hopkins syndrome; clemastine; myelination; sobetirome.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
T.S.S. is a founder and adviser to Autobahn Therapeutics. T.S.S. and T.B. are inventors of licensed patent applications claiming CNS-penetrating thyromimetics. All other authors declare no competing interests.
Figures

Similar articles
-
Clemastine Induces an Impairment in Developmental Myelination.Front Cell Dev Biol. 2022 Mar 17;10:841548. doi: 10.3389/fcell.2022.841548. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372341 Free PMC article.
-
A myelin-related transcriptomic profile is shared by Pitt-Hopkins syndrome models and human autism spectrum disorder.Nat Neurosci. 2020 Mar;23(3):375-385. doi: 10.1038/s41593-019-0578-x. Epub 2020 Feb 3. Nat Neurosci. 2020. PMID: 32015540 Free PMC article.
-
Molecular and Cellular Function of Transcription Factor 4 in Pitt-Hopkins Syndrome.Dev Neurosci. 2021;43(3-4):159-167. doi: 10.1159/000516666. Epub 2021 Jun 16. Dev Neurosci. 2021. PMID: 34134113 Free PMC article. Review.
-
Evaluation of Nav1.8 as a therapeutic target for Pitt Hopkins Syndrome.Mol Psychiatry. 2023 Jan;28(1):76-82. doi: 10.1038/s41380-022-01811-4. Epub 2022 Oct 12. Mol Psychiatry. 2023. PMID: 36224259 Free PMC article. Review.
-
Functional consequences of TCF4 missense substitutions associated with Pitt-Hopkins syndrome, mild intellectual disability, and schizophrenia.J Biol Chem. 2021 Dec;297(6):101381. doi: 10.1016/j.jbc.2021.101381. Epub 2021 Nov 6. J Biol Chem. 2021. PMID: 34748727 Free PMC article.
Cited by
-
Transcription factor 4 expression in the developing non-human primate brain: a comparative analysis with the mouse brain.Front Neuroanat. 2024 Oct 22;18:1478689. doi: 10.3389/fnana.2024.1478689. eCollection 2024. Front Neuroanat. 2024. PMID: 39502395 Free PMC article.
-
Utilizing hiPSC-derived oligodendrocytes to study myelin pathophysiology in neuropsychiatric and neurodegenerative disorders.Front Cell Neurosci. 2024 Jan 11;17:1322813. doi: 10.3389/fncel.2023.1322813. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38273973 Free PMC article. Review.
-
Insights on therapeutic potential of clemastine in neurological disorders.Front Mol Neurosci. 2023 Sep 28;16:1279985. doi: 10.3389/fnmol.2023.1279985. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37840769 Free PMC article. Review.
-
Functional myelin in cognition and neurodevelopmental disorders.Cell Mol Life Sci. 2024 Apr 13;81(1):181. doi: 10.1007/s00018-024-05222-2. Cell Mol Life Sci. 2024. PMID: 38615095 Free PMC article. Review.
-
A novel human organoid model system reveals requirement of TCF4 for oligodendroglial differentiation.Life Sci Alliance. 2025 Mar 28;8(6):e202403102. doi: 10.26508/lsa.202403102. Print 2025 Jun. Life Sci Alliance. 2025. PMID: 40155049 Free PMC article.

