Using a smartwatch and smartphone to assess early Parkinson's disease in the WATCH-PD study
- PMID: 37069193
- PMCID: PMC10108794
- DOI: 10.1038/s41531-023-00497-x
Using a smartwatch and smartphone to assess early Parkinson's disease in the WATCH-PD study
Abstract
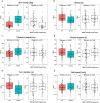
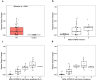
Digital health technologies can provide continuous monitoring and objective, real-world measures of Parkinson's disease (PD), but have primarily been evaluated in small, single-site studies. In this 12-month, multicenter observational study, we evaluated whether a smartwatch and smartphone application could measure features of early PD. 82 individuals with early, untreated PD and 50 age-matched controls wore research-grade sensors, a smartwatch, and a smartphone while performing standardized assessments in the clinic. At home, participants wore the smartwatch for seven days after each clinic visit and completed motor, speech and cognitive tasks on the smartphone every other week. Features derived from the devices, particularly arm swing, the proportion of time with tremor, and finger tapping, differed significantly between individuals with early PD and age-matched controls and had variable correlation with traditional assessments. Longitudinal assessments will inform the value of these digital measures for use in future clinical trials.
© 2023. The Author(s).
Conflict of interest statement
Dr. Adams has received compensation for consulting services from VisualDx and the Huntington Study Group; and research support from Biogen, Biosensics, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, NeuroNext Network, and Safra Foundation. Ms. Tairmae Kangarloo, Dr. Brian Tracey, Dr. Dmitri Volfson, Dr. Neta Zach, and Dr. Robert Latzman are employees of and own stock in Takeda Pharmaceuticals, Inc. Dr. Josh Cosman is an employee of and owns stock in AbbVie Pharmaceuticals. Dr. Jeremey Edgerton, Dr. Krishna Praneeth Kilambi, and Katherine Fisher are employees of and own stock in Biogen Inc. Dr. Peter R. Bergethon was an employee of Biogen during a portion of this study. He has no conflicts or interests at the present time. Dr. Dorsey has received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Bial-Biotech Investments, Inc., Biogen, Boehringer Ingelheim, California Pacific Medical Center, Caraway Therapeutics, Curasen Therapeutics, Denali Therapeutics, Eli Lilly, Genentech/Roche, Grand Rounds, Huntington Study Group, Informa Pharma Consulting, Karger Publications, LifeSciences Consultants, MCM Education, Mediflix, Medopad, Medrhythms, Merck, Michael J. Fox Foundation, NACCME, Neurocrine, NeuroDerm, NIH, Novartis, Origent Data Sciences, Otsuka, Physician’s Education Resource, Praxis, PRIME Education, Roach, Brown, McCarthy & Gruber, Sanofi, Seminal Healthcare, Spark, Springer Healthcare, Sunovion Pharma, Theravance, Voyager and WebMD; research support from Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation, and Wave Life Sciences; editorial services for Karger Publications; stock in Included Health and in Mediflix, and ownership interests in SemCap. Ms. Kostrzebski holds stock in Apple, Inc. Dr. Espay has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Neuroderm, Neurocrine, Amneal, Acadia, Acorda, Bexion, Kyowa Kirin, Sunovion, Supernus (formerly, USWorldMeds), Avion Pharmaceuticals, and Herantis Pharma; personal compensation as honoraria for speakership for Avion; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. He cofounded REGAIN Therapeutics (a biotech start-up developing nonaggregating peptide analogues as replacement therapies for neurodegenerative diseases) and is co-owner of a patent that covers synthetic soluble nonaggregating peptide analogues as replacement treatments in proteinopathies. Dr. Spindler has received compensation for consulting services from Medtronic, and clinical trial funding from Abbvie, Abbott, US WorldMeds, Praxis, and Takeda. Dr. Wyant receives research funding from the National Institutes of Health/National Institute of Neurological Disorders and Stroke NeuroNEXT Network, Eli Lilly, and the Farmer Family Foundation. She also receives royalties from UpToDate. Joan Severson, Allen Best, David Anderson, Michael Merickel, Daniel Jackson Amato, and Brian Severson are employees of Clinical Ink, who acquired the BrainBaseline Platform in 2021 from Digital Artefacts. Joan Severson and Allen Best have financial interests in Clinical Ink.
Figures



References
-
- Dorsey, E. R., Sherer, T., Okun, M. S. & Bloem, B. R. Ending Parkinson’s Disease: A Prescription for Action. (PublicAffairs, 2020).

