Improvement of muscle strength in specific muscular regions in nusinersen-treated adult patients with 5q-spinal muscular atrophy
- PMID: 37069197
- PMCID: PMC10107562
- DOI: 10.1038/s41598-023-31617-5
Improvement of muscle strength in specific muscular regions in nusinersen-treated adult patients with 5q-spinal muscular atrophy
Abstract
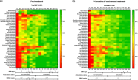
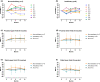
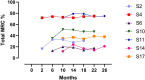
Real-world data have shown mild improvement of overall motor function in adult patients treated with nusinersen, the first approved therapy for 5q-spinal muscular atrophy (SMA). However, knowledge about preferably targeted muscle functions is sparse. The aim of this study was to evaluate strength of distinct muscles and body regions in adult SMA patients in the early course of nusinersen therapy. 72 muscles of 15 patients were tested on the Medical Research Council (MRC) 0-10 scale (translated into MRC %) from nusinersen start to 14 months of treatment. The whole body muscular strength improved slightly or remained stable in 80% of SMA patients with a median improvement of + 2%. However, relevant increases of muscle strength of distinct regions were identified in the proximal upper limbs and shoulder girdle (median + 8%) and in muscle groups with a preserved function pre-treatment, even in more advanced diseased SMA patients. MRC grading was additionally performed in seven patients enrolled during ongoing treatment. Here, further improvement of muscle strength until month 18-26 was seen with the highest increases in the proximal upper and lower limbs. Our findings suggest that sole evaluation of the overall muscle strength might underestimate nusinersen therapy benefits.
© 2023. The Author(s).
Conflict of interest statement
O.S.-K. has received honoraria as a speaker/consultant and/or funding for travel expenses from the German Neuromuscular Society “Deutsche Gesellschaft fuer Muskelkranke (DGM e.V.), Novartis, Biogen GmbH, Biermann Verlag GmbH, and MK + S—Medizin, Kommunikation & Service GmbH. H.A.S. and G.W. report no conflicts of interest. M.K. and G.R. received travel cost compensations from Biogen GmbH. S.P. received honoraria as a speaker/consultant from Biogen GmbH, Roche, Novartis, Teva, Cytoki-netics Inc., and Desitin; and grants from DGM e.V, Federal Ministry of Education and Research, German Israeli Foundation for Scientific Research and Development, EU Joint Programme for Neurodegenerative Disease Research. A.O. received honoraria as a speaker/consultant from Biogen. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Nusinersen: A Review in 5q Spinal Muscular Atrophy.CNS Drugs. 2021 Dec;35(12):1317-1328. doi: 10.1007/s40263-021-00878-x. Epub 2021 Nov 30. CNS Drugs. 2021. PMID: 34850360 Free PMC article.
-
Effect of Discontinuation of Nusinersen Treatment in Long-Standing SMA3.J Neuromuscul Dis. 2021;8(4):537-542. doi: 10.3233/JND-210644. J Neuromuscul Dis. 2021. PMID: 33682724
-
Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4.J Neurol. 2021 Mar;268(3):923-935. doi: 10.1007/s00415-020-10223-9. Epub 2020 Sep 15. J Neurol. 2021. PMID: 32935160
-
Nusinersen: A Treatment for Spinal Muscular Atrophy.Ann Pharmacother. 2019 Jan;53(1):61-69. doi: 10.1177/1060028018789956. Epub 2018 Jul 16. Ann Pharmacother. 2019. PMID: 30008228 Review.
-
Nusinersen: A Review in 5q Spinal Muscular Atrophy.CNS Drugs. 2018 Jul;32(7):689-696. doi: 10.1007/s40263-018-0545-1. CNS Drugs. 2018. PMID: 30027400 Review.
Cited by
-
Consensus from the Brazilian Academy of Neurology for the diagnosis, genetic counseling, and use of disease-modifying therapies in 5q spinal muscular atrophy.Arq Neuropsiquiatr. 2024 Jan;82(1):1-18. doi: 10.1055/s-0044-1779503. Epub 2024 Feb 5. Arq Neuropsiquiatr. 2024. PMID: 38316428 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

