Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies
- PMID: 37069229
- PMCID: PMC10310771
- DOI: 10.1038/s41423-023-01019-8
Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies
Abstract
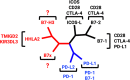
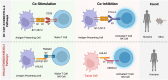
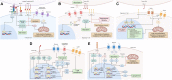
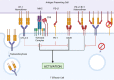
The B7/CD28 families of immune checkpoints play vital roles in negatively or positively regulating immune cells in homeostasis and various diseases. Recent basic and clinical studies have revealed novel biology of the B7/CD28 families and new therapeutics for cancer therapy. In this review, we discuss the newly discovered KIR3DL3/TMIGD2/HHLA2 pathways, PD-1/PD-L1 and B7-H3 as metabolic regulators, the glycobiology of PD-1/PD-L1, B7x (B7-H4) and B7-H3, and the recently characterized PD-L1/B7-1 cis-interaction. We also cover the tumor-intrinsic and -extrinsic resistance mechanisms to current anti-PD-1/PD-L1 and anti-CTLA-4 immunotherapies in clinical settings. Finally, we review new immunotherapies targeting B7-H3, B7x, PD-1/PD-L1, and CTLA-4 in current clinical trials.
Keywords: B7 family; glycobiology; immune checkpoints; metabolic regulators; therapy resistance.
© 2023. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
XZ is the scientific co-founder of NextPoint Therapeutics. The other authors declare no competing interests.
Figures


Similar articles
-
The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3.Immunol Rev. 2017 Mar;276(1):26-39. doi: 10.1111/imr.12521. Immunol Rev. 2017. PMID: 28258693 Free PMC article. Review.
-
KIR3DL3-HHLA2 and TMIGD2-HHLA2 pathways: The dual role of HHLA2 in immune responses and its potential therapeutic approach for cancer immunotherapy.J Adv Res. 2023 May;47:137-150. doi: 10.1016/j.jare.2022.07.013. Epub 2022 Aug 4. J Adv Res. 2023. PMID: 35933091 Free PMC article. Review.
-
PD-L1 and B7-1 Cis-Interaction: New Mechanisms in Immune Checkpoints and Immunotherapies.Trends Mol Med. 2021 Mar;27(3):207-219. doi: 10.1016/j.molmed.2020.10.004. Epub 2020 Nov 13. Trends Mol Med. 2021. PMID: 33199209 Free PMC article. Review.
-
Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma.Cancer Med. 2016 Aug;5(8):1810-20. doi: 10.1002/cam4.754. Epub 2016 Jun 12. Cancer Med. 2016. PMID: 27292320 Free PMC article.
-
HHLA2 immune-regulatory roles in cancer.Biomed Pharmacother. 2023 Jun;162:114639. doi: 10.1016/j.biopha.2023.114639. Epub 2023 Apr 1. Biomed Pharmacother. 2023. PMID: 37011487 Review.
Cited by
-
Unlocking the potential of HHLA2: identifying functional immune infiltrating cells in the tumor microenvironment and predicting clinical outcomes in laryngeal squamous cell carcinoma.Cancer Immunol Immunother. 2024 Aug 6;73(10):207. doi: 10.1007/s00262-024-03791-6. Cancer Immunol Immunother. 2024. PMID: 39105870 Free PMC article.
-
Acquired resistance in cancer: towards targeted therapeutic strategies.Nat Rev Cancer. 2025 Aug;25(8):613-633. doi: 10.1038/s41568-025-00824-9. Epub 2025 Jun 3. Nat Rev Cancer. 2025. PMID: 40461793 Free PMC article. Review.
-
The Impact of Laparoscopic Appendectomy and Open Appendectomy on B7-H3-Mediated Intrinsic Immune Response in Children with Acute Suppurative Appendicitis.J Inflamm Res. 2024 Mar 11;17:1577-1587. doi: 10.2147/JIR.S446199. eCollection 2024. J Inflamm Res. 2024. PMID: 38495342 Free PMC article.
-
B7-H3 in glioblastoma and beyond: significance and therapeutic strategies.Front Immunol. 2024 Nov 25;15:1495283. doi: 10.3389/fimmu.2024.1495283. eCollection 2024. Front Immunol. 2024. PMID: 39664380 Free PMC article. Review.
-
The immune regulatory function of B7-H3 in malignancy: spotlight on the IFN-STAT1 axis and regulation of tumor-associated macrophages.Immunol Res. 2024 Aug;72(4):526-537. doi: 10.1007/s12026-024-09458-9. Epub 2024 Jan 24. Immunol Res. 2024. PMID: 38265550 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

