Distinct astrocytic modulatory roles in sensory transmission during sleep, wakefulness, and arousal states in freely moving mice
- PMID: 37069258
- PMCID: PMC10110578
- DOI: 10.1038/s41467-023-37974-z
Distinct astrocytic modulatory roles in sensory transmission during sleep, wakefulness, and arousal states in freely moving mice
Abstract
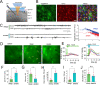
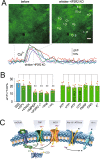
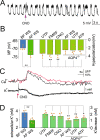
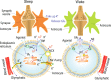
Despite extensive research on astrocytic Ca2+ in synaptic transmission, its contribution to the modulation of sensory transmission during different brain states remains largely unknown. Here, by using two-photon microscopy and whole-cell recordings, we show two distinct astrocytic Ca2+ signals in the murine barrel cortex: a small, long-lasting Ca2+ increase during sleep and a large, widespread but short-lasting Ca2+ spike when aroused. The large Ca2+ wave in aroused mice was inositol trisphosphate (IP3)-dependent, evoked by the locus coeruleus-norepinephrine system, and enhanced sensory input, contributing to reliable sensory transmission. However, the small Ca2+ transient was IP3-independent and contributed to decreased extracellular K+, hyperpolarization of the neurons, and suppression of sensory transmission. These events respond to different pharmacological inputs and contribute to distinct sleep and arousal functions by modulating the efficacy of sensory transmission. Together, our data demonstrate an important function for astrocytes in sleep and arousal states via astrocytic Ca2+ waves.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

