Antiviral neutralizing antibodies: from in vitro to in vivo activity
- PMID: 37069260
- PMCID: PMC10108814
- DOI: 10.1038/s41577-023-00858-w
Antiviral neutralizing antibodies: from in vitro to in vivo activity
Abstract
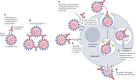
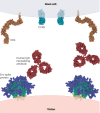
Neutralizing antibodies (nAbs) are being increasingly used as passive antiviral reagents in prophylactic and therapeutic modalities and to guide viral vaccine design. In vivo, nAbs can mediate antiviral functions through several mechanisms, including neutralization, which is defined by in vitro assays in which nAbs block viral entry to target cells, and antibody effector functions, which are defined by in vitro assays that evaluate nAbs against viruses and infected cells in the presence of effector systems. Interpreting in vivo results in terms of these in vitro assays is challenging but important in choosing optimal passive antibody and vaccine strategies. Here, I review findings from many different viruses and conclude that, although some generalizations are possible, deciphering the relative contributions of different antiviral mechanisms to the in vivo efficacy of antibodies currently requires consideration of individual antibody-virus interactions.
© 2023. Springer Nature Limited.
Conflict of interest statement
The author declares no competing interests.
Figures

References
-
- Dimmock NJ. Update on the neutralization of animal viruses. Rev. Med. Virol. 1995;5:165–179. doi: 10.1002/rmv.1980050306. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

