Targeting IL-6 trans-signalling: past, present and future prospects
- PMID: 37069261
- PMCID: PMC10108826
- DOI: 10.1038/s41577-023-00856-y
Targeting IL-6 trans-signalling: past, present and future prospects
Abstract
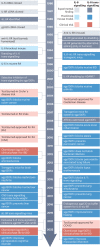
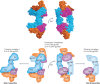
Interleukin-6 (IL-6) is a key immunomodulatory cytokine that affects the pathogenesis of diverse diseases, including autoimmune diseases, chronic inflammatory conditions and cancer. Classical IL-6 signalling involves the binding of IL-6 to the membrane-bound IL-6 receptor α-subunit (hereafter termed 'mIL-6R') and glycoprotein 130 (gp130) signal-transducing subunit. By contrast, in IL-6 trans-signalling, complexes of IL-6 and the soluble form of IL-6 receptor (sIL-6R) signal via membrane-bound gp130. A third mode of IL-6 signalling - known as cluster signalling - involves preformed complexes of membrane-bound IL-6-mIL-6R on one cell activating gp130 subunits on target cells. Antibodies and small molecules have been developed that block all three forms of IL-6 signalling, but in the past decade, IL-6 trans-signalling has emerged as the predominant pathway by which IL-6 promotes disease pathogenesis. The first selective inhibitor of IL-6 trans-signalling, sgp130, has shown therapeutic potential in various preclinical models of disease and olamkicept, a sgp130Fc variant, had promising results in phase II clinical studies for inflammatory bowel disease. Technological developments have already led to next-generation sgp130 variants with increased affinity and selectivity towards IL-6 trans-signalling, along with indirect strategies to block IL-6 trans-signalling. Here, we summarize our current understanding of the biological outcomes of IL-6-mediated signalling and the potential for targeting this pathway in the clinic.
© 2023. Springer Nature Limited.
Conflict of interest statement
C.G. has received a research grant from Corvidia Therapeutics (Waltham, MA, USA) and has acted as a consultant/speaker for AbbVie and NovoNordisk. J.M.M. and J.S. declare that they have applied for a patent covering cs130Fc and c19s130Fc. J.M.M. has acted as a consultant for Ferring Pharmaceuticals. S.R.-J. has acted as a consultant and speaker for AbbVie, Chugai, Genentech Roche, Regeneron, Pfizer and Sanofi. He also declares that he is an inventor on patents owned by CONARIS Research Institute, which develops the sgp130Fc protein olamkicept together with Ferring Pharmaceuticals and I-Mab Biopharma. S.R.-J. has stock ownership in CONARIS. B.J.J. declares no competing interests.
Figures




References
-
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon β2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl Acad. Sci. USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

