Developmental loss of ErbB4 in PV interneurons disrupts state-dependent cortical circuit dynamics
- PMID: 37069344
- PMCID: PMC10618960
- DOI: 10.1038/s41380-023-02066-3
Developmental loss of ErbB4 in PV interneurons disrupts state-dependent cortical circuit dynamics
Abstract
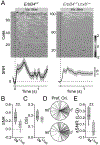
GABAergic inhibition plays an important role in the establishment and maintenance of cortical circuits during development. Neuregulin 1 (Nrg1) and its interneuron-specific receptor ErbB4 are key elements of a signaling pathway critical for the maturation and proper synaptic connectivity of interneurons. Using conditional deletions of the ERBB4 gene in mice, we tested the role of this signaling pathway at two developmental timepoints in parvalbumin-expressing (PV) interneurons, the largest subpopulation of cortical GABAergic cells. Loss of ErbB4 in PV interneurons during embryonic, but not late postnatal development leads to alterations in the activity of excitatory and inhibitory cortical neurons, along with severe disruption of cortical temporal organization. These impairments emerge by the end of the second postnatal week, prior to the complete maturation of the PV interneurons themselves. Early loss of ErbB4 in PV interneurons also results in profound dysregulation of excitatory pyramidal neuron dendritic architecture and a redistribution of spine density at the apical dendritic tuft. In association with these deficits, excitatory cortical neurons exhibit normal tuning for sensory inputs, but a loss of state-dependent modulation of the gain of sensory responses. Together these data support a key role for early developmental Nrg1/ErbB4 signaling in PV interneurons as a powerful mechanism underlying the maturation of both the inhibitory and excitatory components of cortical circuits.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures


References
-
- Cossart R The maturation of cortical interneuron diversity: how multiple developmental journeys shape the emergence of proper network function. Curr Opin Neurobiol. 2011;21:160–8. - PubMed
-
- Modol L, Sousa VH, Malvache A, Tressard T, Baude A, Cossart R. Spatial embryonic origin delineates GABAergic hub neurons driving network dynamics in the developing entorhinal cortex. Cereb Cortex. 2017;27:4649–61. - PubMed
-
- Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

