Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial
- PMID: 37069359
- PMCID: PMC10115633
- DOI: 10.1038/s41591-023-02273-z
Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial
Abstract
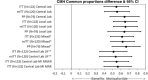
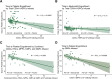
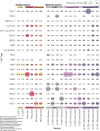
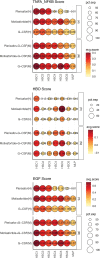
Autologous hematopoietic stem cell transplantation (ASCT) improves survival in multiple myeloma (MM). However, many individuals are unable to collect optimal CD34+ hematopoietic stem and progenitor cell (HSPC) numbers with granulocyte colony-stimulating factor (G-CSF) mobilization. Motixafortide is a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity. The GENESIS trial was a prospective, phase 3, double-blind, placebo-controlled, multicenter study with the objective of assessing the superiority of motixafortide + G-CSF over placebo + G-CSF to mobilize HSPCs for ASCT in MM. The primary endpoint was the proportion of patients collecting ≥6 × 106 CD34+ cells kg-1 within two apheresis procedures; the secondary endpoint was to achieve this goal in one apheresis. A total of 122 adult patients with MM undergoing ASCT were enrolled at 18 sites across five countries and randomized (2:1) to motixafortide + G-CSF or placebo + G-CSF for HSPC mobilization. Motixafortide + G-CSF enabled 92.5% to successfully meet the primary endpoint versus 26.2% with placebo + G-CSF (odds ratio (OR) 53.3, 95% confidence interval (CI) 14.12-201.33, P < 0.0001). Motixafortide + G-CSF also enabled 88.8% to meet the secondary endpoint versus 9.5% with placebo + G-CSF (OR 118.0, 95% CI 25.36-549.35, P < 0.0001). Motixafortide + G-CSF was safe and well tolerated, with the most common treatment-emergent adverse events observed being transient, grade 1/2 injection site reactions (pain, 50%; erythema, 27.5%; pruritis, 21.3%). In conclusion, motixafortide + G-CSF mobilized significantly greater CD34+ HSPC numbers within two apheresis procedures versus placebo + G-CSF while preferentially mobilizing increased numbers of immunophenotypically and transcriptionally primitive HSPCs. Trial Registration: ClinicalTrials.gov , NCT03246529.
© 2023. The Author(s).
Conflict of interest statement
Z.D.C.: research funding, BioLineRx; M.P.R.: no competing interests. R.G.J.: consulting fees, WUGEN. K.S.-G.: research funding, BioLineRx, Caelum Biosciences, GlaxoSmithKline, GlycoMimetics, Ionis Pharmaceuticals, Janssen Pharmaceuticals, Millennium Pharmaceuticals (Takeda), Sanofi; equity stock/ownership, Abbott, AbbVie; consulting Fees, GlaxoSmithKline; board or advisory committee membership, GlaxoSmithKline; speaking fees, Janssen Pharmaceuticals; expert witness, Celgene. G.A.M., M.M., P.S.: no competing interests. I.A.: consulting fees and board or advisory committee membership, Janssen, Celgene, Novartis, Pfizer, Takeda, Roche. D.S: consulting fees, Janssen, AbbVie, Sanofi, GlaxoSmithKline, BristolMyersSquibb, Pfizer; board or advisory committee membership, Janssen, Sanofi, GlaxoSmithKline. D.P.: no competing interests. I.M.: research funding, BioLineRx. G.M.J.: no competing interests. G.M.: research funding, AbbVie, Janssen-Cilag; consulting fees, AbbVie, Amgen, Bristol-Myers-Squibb/Celgene, Janssen-Cilag, Krka, Novartis, Roche, Takeda. M.L.P.C.: no competing interests. U.H.: consulting fees, Sanofi-Adventis. J.H.: no competing interests. M.H.Q.: research funding, Janssen Pharmaceuticals, BioLineRx, Angiocrrine, Neximmune. N.H.: board or advisory committee membership, Incyte, Kite/Gilead, American Gene Technologies. T.L., I.G.-C.: no competing interests. A.V.-H., E.S., I.G.-K., I.G., D.I., L.S.-D.: employment, BioLineRx. S.K., F.G.: no competing interests. M.A.S.: research funding, Incyte, Cellect, Inc., Equillium, Inc., Fortis, Seattle Genetics, Amgen, Celgene, PBD, Inc., Sanofi Genzyme, Janssen; consulting fees and board or advisory committee membership, Advarra, Amgen, Astellas, CareDx, Dova pharmaceuticals, Equillium, Inc., FlatIron, Inc., GlaxoSmithKline, Gilead Sciences, Inc., Incyte, Inmagene Bio, Janssen, Novo Nordisk, Partners Therapeutics, Pfizer, Sanofi Genzyme, StemLine. R.V.: no competing interests. J.F.D.: equity stock/ownership, Magenta Therapeutics, WUGEN; consulting fees, Incyte, RiverVest Venture Partners; board or advisory committee membership, Cellworks Group, RiverVest Venture Partners, Magenta; research funding, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, Incyte, BioLineRx, WUGEN; speaking fees, Incyte; patents (nos. PCT/US2019/035010 and PCT/US2019/035052), WUGEN, Magenta.
Figures








References
-
- Fermand JP, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J. Clin. Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

