Drug-related problems reported by patients with rheumatic diseases: an observational study
- PMID: 37069634
- PMCID: PMC10111673
- DOI: 10.1186/s41927-023-00326-x
Drug-related problems reported by patients with rheumatic diseases: an observational study
Abstract
Background: Drug-related problems can negatively influence treatment outcome and well-being for patients with rheumatic diseases. Thus, it is important to support patients in preventing or resolving drug-related problems as quickly as possible. To effectively develop interventions for this purpose, knowledge on the frequency and character of drug-related problems is needed. Therefore, this study aims to quantify and characterize drug-related problems reported by patients with inflammatory rheumatic diseases along their treatment process.
Methods: A prospective observational study was conducted in a Dutch outpatient pharmacy. Adult patients with rheumatic diseases that were prescribed medication by a rheumatologist were questioned about experienced DRPs by telephone 4 times in 8 weeks using a structured interview-guide. Patient-reported DRPs were scored on uniqueness (i.e., if a specific DRP was reported in multiple interviews by one individual, this was counted as one unique DRP) and were categorized using a classification for patient-reported DRPs and analysed descriptively.
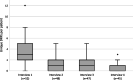
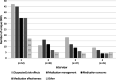
Results: In total, 52 participants (median age 68 years (interquartile range (IQR) 62-74), 52% male) completed 192 interviews with 45 (87%) participants completing all 4 interviews. The majority of patients (65%) were diagnosed with rheumatoid arthritis. Patients reported a median number of 3 (IQR 2-5) unique DRPs during interview 1. In subsequent interviews, patients reported median numbers of 1 (IQR 0-2), 1 (IQR 0-2) and 0 (IQR 0-1) unique DRPs for interviews 2-4 respectively. Participants reported a median number of 5 (IQR 3-9) unique DRPs over all completed interviews. Unique patient-reported DRPs were most frequently categorized into (suspected) side effects (28%), medication management (e.g., medication administering or adherence) (26%), medication concerns (e.g., concerns regarding long-term side-effects or effectiveness) (19%) and medication effectiveness (17%).
Conclusions: Patients with rheumatic diseases report various unique DRPs with intervals as short as two weeks. These patients might therefore benefit from more continuous support in-between contact moments with their healthcare provider.
Keywords: Drug-related problems; Observational; Patient-reported; Rheumatic diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Clinical medication reviews in elderly patients with polypharmacy: a cross-sectional study on drug-related problems in the Netherlands.Int J Clin Pharm. 2016 Feb;38(1):46-53. doi: 10.1007/s11096-015-0199-8. Epub 2015 Nov 23. Int J Clin Pharm. 2016. PMID: 26597955 Free PMC article.
-
Development of a Predictive Model for Drug-Related Problems in Kidney Transplant Recipients.Pharmacotherapy. 2017 Feb;37(2):159-169. doi: 10.1002/phar.1886. Epub 2017 Feb 3. Pharmacotherapy. 2017. PMID: 27997676
-
Contribution of Patient Interviews as Part of a Comprehensive Approach to the Identification of Drug-Related Problems on Geriatric Wards.Drugs Aging. 2018 Jul;35(7):665-675. doi: 10.1007/s40266-018-0557-z. Drugs Aging. 2018. PMID: 29916139
-
Systematic review of the prevalence and nature of drug-related problems in paediatric patients.J Clin Pharm Ther. 2022 Jun;47(6):776-782. doi: 10.1111/jcpt.13606. Epub 2022 Jan 30. J Clin Pharm Ther. 2022. PMID: 35098566 Free PMC article.
-
Evaluation of Pharmacist Medication Review Service in an Outpatient Heart Failure Clinic.J Pharm Pract. 2020 Dec;33(6):820-826. doi: 10.1177/0897190019842696. Epub 2019 May 5. J Pharm Pract. 2020. PMID: 31057060 Review.
Cited by
-
Addressing Glucocorticoid-Related Problems with the Clinical Pharmacist Collaboration in Rheumatology Practice: A Prospective Follow-Up Study.Rheumatol Ther. 2024 Aug;11(4):1043-1055. doi: 10.1007/s40744-024-00692-z. Epub 2024 Jun 26. Rheumatol Ther. 2024. PMID: 38926304 Free PMC article.
-
Factors Influencing the Intentions of Patients With Inflammatory Rheumatic Diseases to Use a Digital Human for Medication Information: Qualitative Study.J Med Internet Res. 2025 Mar 13;27:e57697. doi: 10.2196/57697. J Med Internet Res. 2025. PMID: 40080817 Free PMC article.
-
Drug-related problems experienced by rheumatoid arthritis patients during the first three months of methotrexate use: a longitudinal observational study.Int J Clin Pharm. 2025 Jun;47(3):884-890. doi: 10.1007/s11096-025-01904-4. Epub 2025 Apr 3. Int J Clin Pharm. 2025. PMID: 40178797
-
Fatigue patterns surrounding biologic disease-modifying antirheumatic drug injection in patients with an inflammatory rheumatic disease: an ecological momentary assessment study.Rheumatol Int. 2025 Jan 11;45(1):24. doi: 10.1007/s00296-024-05779-y. Rheumatol Int. 2025. PMID: 39797990 Free PMC article.
-
Factors Influencing Preferences of Patients With Rheumatic Diseases Regarding Telehealth Channels for Support With Medication Use: Qualitative Study.JMIR Form Res. 2023 Jul 20;7:e45086. doi: 10.2196/45086. JMIR Form Res. 2023. PMID: 37471137 Free PMC article.
References
-
- Burkhart PV, Sabaté E, Adherence to long-term therapies: evidence for action. (2003) - PubMed
-
- Vlieland ND, Gardarsdottir H, Bouvy ML, Egberts TCG, van den Bemt BJF. The majority of patients do not store their biologic disease-modifying antirheumatic drugs within the recommended temperature range. Rheumatol (United Kingdom) 2016;55:704–709. - PubMed
LinkOut - more resources
Full Text Sources

