Noninvasive ultrasound stimulation to treat myocarditis through splenic neuro-immune regulation
- PMID: 37069636
- PMCID: PMC10108488
- DOI: 10.1186/s12974-023-02773-2
Noninvasive ultrasound stimulation to treat myocarditis through splenic neuro-immune regulation
Abstract
Background: The cholinergic anti-inflammatory pathway (CAP) has been widely studied to modulate the immune response. Current stimulating strategies are invasive or imprecise. Noninvasive low-intensity pulsed ultrasound (LIPUS) has become increasingly appreciated for targeted neuronal modulation. However, its mechanisms and physiological role on myocarditis remain poorly defined.
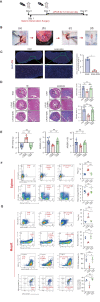
Methods: The mouse model of experimental autoimmune myocarditis was established. Low-intensity pulsed ultrasound was targeted at the spleen to stimulate the spleen nerve. Under different ultrasound parameters, histological tests and molecular biology were performed to observe inflammatory lesions and changes in immune cell subsets in the spleen and heart. In addition, we evaluated the dependence of the spleen nerve and cholinergic anti-inflammatory pathway of low-intensity pulsed ultrasound in treating autoimmune myocarditis in mice through different control groups.
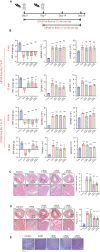
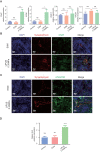
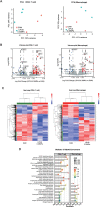
Results: The echocardiography and flow cytometry of splenic or heart infiltrating immune cells revealed that splenic ultrasound could alleviate the immune response, regulate the proportion and function of CD4+ Treg and macrophages by activating cholinergic anti-inflammatory pathway, and finally reduce heart inflammatory injury and improve cardiac remodeling, which is as effective as an acetylcholine receptor agonists GTS-21. Transcriptome sequencing showed significant differential expressed genes due to ultrasound modulation.
Conclusions: It is worth noting that the ultrasound therapeutic efficacy depends greatly on acoustic pressure and exposure duration, and the effective targeting organ was the spleen but not the heart. This study provides novel insight into the therapeutic potentials of LIPUS, which are essential for its future application.
Keywords: CCR2+ macrophage; CD4+ Treg cell; Cholinergic anti-inflammatory pathway; Experimental autoimmune myocarditis; Low-intensity pulsed ultrasound.
© 2023. The Author(s).
Conflict of interest statement
We declare that we have no conflict of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

