D-xylose accelerated death of pentose metabolizing Saccharomyces cerevisiae
- PMID: 37069654
- PMCID: PMC10111712
- DOI: 10.1186/s13068-023-02320-4
D-xylose accelerated death of pentose metabolizing Saccharomyces cerevisiae
Abstract
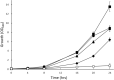
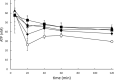
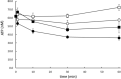
Rapid and effective consumption of D-xylose by Saccharomyces cerevisiae is essential for cost-efficient cellulosic bioethanol production. Hence, heterologous D-xylose metabolic pathways have been introduced into S. cerevisiae. An effective solution is based on a xylose isomerase in combination with the overexpression of the xylulose kinase (Xks1) and all genes of the non-oxidative branch of the pentose phosphate pathway. Although this strain is capable of consuming D-xylose, growth inhibition occurs at higher D-xylose concentrations, even abolishing growth completely at 8% D-xylose. The decreased growth rates are accompanied by significantly decreased ATP levels. A key ATP-utilizing step in D-xylose metabolism is the phosphorylation of D-xylulose by Xks1. Replacement of the constitutive promoter of XKS1 by the galactose tunable promoter Pgal10 allowed the controlled expression of this gene over a broad range. By decreasing the expression levels of XKS1, growth at high D-xylose concentrations could be restored concomitantly with increased ATP levels and high rates of xylose metabolism. These data show that in fermentations with high D-xylose concentrations, too high levels of Xks1 cause a major drain on the cellular ATP levels thereby reducing the growth rate, ultimately causing substrate accelerated death. Hence, expression levels of XKS1 in S. cerevisiae needs to be tailored for the specific growth conditions and robust D-xylose metabolism.
Keywords: ATP; Bioethanol; D-xylose consumption; Xks1 expression; Yeast.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
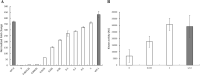

Similar articles
-
Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability.Metab Eng. 2001 Jul;3(3):236-49. doi: 10.1006/mben.2000.0191. Metab Eng. 2001. PMID: 11461146
-
The non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose in Saccharomyces cerevisiae TMB3001.FEMS Yeast Res. 2002 Aug;2(3):277-82. doi: 10.1111/j.1567-1364.2002.tb00095.x. FEMS Yeast Res. 2002. PMID: 12702276
-
Optimal growth and ethanol production from xylose by recombinant Saccharomyces cerevisiae require moderate D-xylulokinase activity.Appl Environ Microbiol. 2003 Jan;69(1):495-503. doi: 10.1128/AEM.69.1.495-503.2003. Appl Environ Microbiol. 2003. PMID: 12514033 Free PMC article.
-
Development of efficient xylose fermentation in Saccharomyces cerevisiae: xylose isomerase as a key component.Adv Biochem Eng Biotechnol. 2007;108:179-204. doi: 10.1007/10_2007_057. Adv Biochem Eng Biotechnol. 2007. PMID: 17846724 Review.
-
Engineering of Pentose Transport in Saccharomyces cerevisiae for Biotechnological Applications.Front Bioeng Biotechnol. 2020 Jan 29;7:464. doi: 10.3389/fbioe.2019.00464. eCollection 2019. Front Bioeng Biotechnol. 2020. PMID: 32064252 Free PMC article. Review.
Cited by
-
Engineering transcriptional regulatory networks for improving second-generation fuel ethanol production in Saccharomyces cerevisiae.Synth Syst Biotechnol. 2024 Oct 28;10(1):207-217. doi: 10.1016/j.synbio.2024.10.006. eCollection 2025. Synth Syst Biotechnol. 2024. PMID: 39558946 Free PMC article. Review.
-
Maltose accumulation-induced cell death in Saccharomyces cerevisiae.FEMS Yeast Res. 2024 Jan 9;24:foae012. doi: 10.1093/femsyr/foae012. FEMS Yeast Res. 2024. PMID: 38565313 Free PMC article.
-
Evaluation of Pyrophosphate-Driven Proton Pumps in Saccharomyces cerevisiae under Stress Conditions.Microorganisms. 2024 Mar 20;12(3):625. doi: 10.3390/microorganisms12030625. Microorganisms. 2024. PMID: 38543676 Free PMC article.
-
Saccharomyces cerevisiae for lignocellulosic ethanol production: a look at key attributes and genome shuffling.Front Bioeng Biotechnol. 2024 Sep 25;12:1466644. doi: 10.3389/fbioe.2024.1466644. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39386039 Free PMC article. Review.
References
-
- Kotter P, Ciriacy M. Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1993;38(6):776–783. doi: 10.1007/BF00167144. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
