Low-intensity pulsed ultrasound promotes mesenchymal stem cell transplantation-based articular cartilage regeneration via inhibiting the TNF signaling pathway
- PMID: 37069673
- PMCID: PMC10111837
- DOI: 10.1186/s13287-023-03296-6
Low-intensity pulsed ultrasound promotes mesenchymal stem cell transplantation-based articular cartilage regeneration via inhibiting the TNF signaling pathway
Abstract
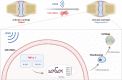
Background: Mesenchymal stem cell (MSC) transplantation therapy is highly investigated for the regenerative repair of cartilage defects. Low-intensity pulsed ultrasound (LIPUS) has the potential to promote chondrogenic differentiation of MSCs. However, its underlying mechanism remains unclear. Here, we investigated the promoting effects and mechanisms underlying LIPUS stimulation on the chondrogenic differentiation of human umbilical cord mesenchymal stem cells (hUC-MSCs) and further evaluated its regenerative application value in articular cartilage defects in rats.
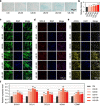
Methods: LIPUS was applied to stimulate cultured hUC-MSCs and C28/I2 cells in vitro. Immunofluorescence staining, qPCR analysis, and transcriptome sequencing were used to detect mature cartilage-related markers of gene and protein expression for a comprehensive evaluation of differentiation. Injured articular cartilage rat models were established for further hUC-MSC transplantation and LIPUS stimulation in vivo. Histopathology and H&E staining were used to evaluate the repair effects of the injured articular cartilage with LIPUS stimulation.
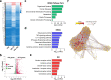
Results: The results showed that LIPUS stimulation with specific parameters effectively promoted the expression of mature cartilage-related genes and proteins, inhibited TNF-α gene expression in hUC-MSCs, and exhibited anti-inflammation in C28/I2 cells. In addition, the articular cartilage defects of rats were significantly repaired after hUC-MSC transplantation and LIPUS stimulation.
Conclusions: Taken together, LIPUS stimulation could realize articular cartilage regeneration based on hUC-MSC transplantation due to the inhibition of the TNF signaling pathway, which is of clinical value for the relief of osteoarthritis.
Keywords: Articular cartilage regeneration; Chondrogenic differentiation; Low-intensity pulsed ultrasound (LIPUS); Mesenchymal stem cells (MSCs); TNF signaling pathway.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Low-Intensity Pulsed Ultrasound Promotes Autophagy-Mediated Migration of Mesenchymal Stem Cells and Cartilage Repair.Cell Transplant. 2021 Jan-Dec;30:963689720986142. doi: 10.1177/0963689720986142. Cell Transplant. 2021. PMID: 33412895 Free PMC article.
-
Intra-articular injection of hUC-MSCs expressing miR-140-5p induces cartilage self-repairing in the rat osteoarthritis.J Bone Miner Metab. 2020 May;38(3):277-288. doi: 10.1007/s00774-019-01055-3. Epub 2019 Nov 23. J Bone Miner Metab. 2020. PMID: 31760502
-
Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats.Stem Cell Res Ther. 2017 Dec 19;8(1):283. doi: 10.1186/s13287-017-0739-3. Stem Cell Res Ther. 2017. PMID: 29258619 Free PMC article.
-
Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells.Stem Cell Res Ther. 2022 May 26;13(1):214. doi: 10.1186/s13287-022-02887-z. Stem Cell Res Ther. 2022. PMID: 35619156 Free PMC article. Review.
-
Repair of injured articular and growth plate cartilage using mesenchymal stem cells and chondrogenic gene therapy.Curr Stem Cell Res Ther. 2006 May;1(2):213-29. doi: 10.2174/157488806776956904. Curr Stem Cell Res Ther. 2006. PMID: 18220868 Review.
Cited by
-
Emerging Strategies in Cartilage Repair and Joint Preservation.Medicina (Kaunas). 2024 Dec 27;61(1):24. doi: 10.3390/medicina61010024. Medicina (Kaunas). 2024. PMID: 39859006 Free PMC article. Review.
-
LIPUS promotes osteogenic differentiation of rat BMSCs and osseointegration of dental implants by regulating ITGA11 and focal adhesion pathway.BMC Oral Health. 2025 Jan 4;25(1):22. doi: 10.1186/s12903-024-05411-2. BMC Oral Health. 2025. PMID: 39755586 Free PMC article.
-
Knowledge mapping and global trends in the field of low-intensity pulsed ultrasound and endocrine and metabolic diseases: a bibliometric and visual analysis from 2012 to 2022.Front Endocrinol (Lausanne). 2023 Sep 5;14:1237864. doi: 10.3389/fendo.2023.1237864. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37732128 Free PMC article.
-
Ultrasound-triggered three dimensional hyaluronic acid hydrogel promotes in vitro and in vivo reprogramming into induced pluripotent stem cells.Bioact Mater. 2024 May 9;38:331-345. doi: 10.1016/j.bioactmat.2024.05.011. eCollection 2024 Aug. Bioact Mater. 2024. PMID: 38764447 Free PMC article.
-
Deciphering the Role of LncRNAs in Osteoarthritis: Inflammatory Pathways Unveiled.J Inflamm Res. 2024 Sep 20;17:6563-6581. doi: 10.2147/JIR.S489682. eCollection 2024. J Inflamm Res. 2024. PMID: 39318993 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

