No evidence for schistosome parasite fitness trade-offs in the intermediate and definitive host
- PMID: 37069704
- PMCID: PMC10111729
- DOI: 10.1186/s13071-023-05730-3
No evidence for schistosome parasite fitness trade-offs in the intermediate and definitive host
Abstract
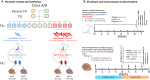
Background: The trematode parasite Schistosoma mansoni uses an aquatic snail intermediate and a vertebrate definitive host to complete its life cycle. We previously showed that a key transmission trait-the number of cercariae larvae shed from infected Biomphalaria spp. snails-varies significantly within and between different parasite populations and is genetically controlled by five loci. We investigated the hypothesis that the success of parasite genotypes showing high propagative fitness in the intermediate snail host may be offset by lower reproductive fitness in the definitive vertebrate host.
Methods: We investigated this trade-off hypothesis by selecting parasite progeny producing high or low number of larvae in the snail and then comparing fitness parameters and virulence in the rodent host. We infected inbred BALB/c mice using two Schistosoma mansoni parasite lines [high shedder (HS) and low shedder (LS) lines] isolated from F2 progeny generated by genetic crosses between SmLE (HS parent) and SmBRE (LS parent) parasites. We used the F3 progeny to infect two populations of inbred Biomphalaria glabrata snails. We then compared life history traits and virulence of these two selected parasite lines in the rodent host to understand pleiotropic effects of genes determining cercarial shedding in parasites infecting the definitive host.
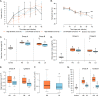
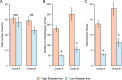
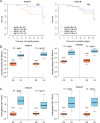
Results: HS parasites shed high numbers of cercariae, which had a detrimental impact on snail physiology (measured by laccase-like activity and hemoglobin rate), regardless of the snail genetic background. In contrast, selected LS parasites shed fewer cercariae and had a lower impact on snail physiology. Similarly, HS worms have a higher reproductive fitness and produced more viable F3 miracidia larvae than LS parasites. This increase in transmission is correlated with an increase in virulence toward the rodent host, characterized by stronger hepato-splenomegaly and hepatic fibrosis.
Conclusions: These experiments revealed that schistosome parasite propagative and reproductive fitness was positively correlated in intermediate and definitive host (positive pleiotropy). Therefore, we rejected our trade-off hypothesis. We also showed that our selected schistosome lines exhibited low and high shedding phenotype regardless of the intermediate snail host genetic background. .
Keywords: Biomphalaria snail host; Genetic crosses; Positive pleiotropy; Rodent host; Schistosoma parasite; Selection; Virulence-transmission trade-offs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Striking differences in virulence, transmission and sporocyst growth dynamics between two schistosome populations.Parasit Vectors. 2019 Oct 16;12(1):485. doi: 10.1186/s13071-019-3741-z. Parasit Vectors. 2019. PMID: 31619284 Free PMC article.
-
Genetic architecture of transmission stage production and virulence in schistosome parasites.Virulence. 2021 Dec;12(1):1508-1526. doi: 10.1080/21505594.2021.1932183. Virulence. 2021. PMID: 34167443 Free PMC article.
-
Genotypic variation in host response to infection affects parasite reproductive rate.Int J Parasitol. 2016 Feb;46(2):123-31. doi: 10.1016/j.ijpara.2015.10.001. Epub 2015 Nov 10. Int J Parasitol. 2016. PMID: 26552016
-
Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: a 2009 assessment.Mol Biochem Parasitol. 2009 May;165(1):8-18. doi: 10.1016/j.molbiopara.2009.01.005. Epub 2009 Jan 22. Mol Biochem Parasitol. 2009. PMID: 19393158 Free PMC article. Review.
-
Are Biomphalaria snails resistant to Schistosoma mansoni?J Helminthol. 2005 Sep;79(3):187-91. doi: 10.1079/joh2005299. J Helminthol. 2005. PMID: 16153311 Review.
Cited by
-
Rapid phenotypic and genotypic change in a laboratory schistosome population.bioRxiv [Preprint]. 2024 Aug 8:2024.08.06.606850. doi: 10.1101/2024.08.06.606850. bioRxiv. 2024. PMID: 39149304 Free PMC article. Preprint.
-
Contribution of parasite and host genotype to immunopathology of schistosome infections.Parasit Vectors. 2024 May 7;17(1):203. doi: 10.1186/s13071-024-06286-6. Parasit Vectors. 2024. PMID: 38711063 Free PMC article.
-
Abundant genetic variation is retained in many laboratory schistosome populations.bioRxiv [Preprint]. 2024 Oct 24:2024.10.21.619418. doi: 10.1101/2024.10.21.619418. bioRxiv. 2024. PMID: 39484487 Free PMC article. Preprint.
-
Rapid phenotypic and genotypic change in a laboratory schistosome population.Res Sq [Preprint]. 2024 Sep 16:rs.3.rs-4869982. doi: 10.21203/rs.3.rs-4869982/v1. Res Sq. 2024. Update in: Parasit Vectors. 2024 Dec 22;17(1):528. doi: 10.1186/s13071-024-06588-9. PMID: 39372934 Free PMC article. Updated. Preprint.
-
Contribution of parasite and host genotype to immunopathology of schistosome infections.bioRxiv [Preprint]. 2024 Jan 13:2024.01.12.574230. doi: 10.1101/2024.01.12.574230. bioRxiv. 2024. Update in: Parasit Vectors. 2024 May 7;17(1):203. doi: 10.1186/s13071-024-06286-6. PMID: 38260613 Free PMC article. Updated. Preprint.
References
-
- Kalbe M, Eizaguirre C, Scharsack JP, Jakobsen PJ. Reciprocal cross infection of sticklebacks with the diphyllobothriidean cestode Schistocephalus solidus reveals consistent population differences in parasite growth and host resistance. Parasit Vectors. 2016;9:130. doi: 10.1186/s13071-016-1419-3. - DOI - PMC - PubMed
-
- Gower CM, Webster JP. Fitness of indirectly transmitted pathogens: restraint and constraint. Evolution. 2004;58:1178–1184. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

