The Transcriptomics of the Human Vein Transformation After Arteriovenous Fistula Anastomosis Uncovers Layer-Specific Remodeling and Hallmarks of Maturation Failure
- PMID: 37069981
- PMCID: PMC10105062
- DOI: 10.1016/j.ekir.2023.01.008
The Transcriptomics of the Human Vein Transformation After Arteriovenous Fistula Anastomosis Uncovers Layer-Specific Remodeling and Hallmarks of Maturation Failure
Abstract
Introduction: The molecular transformation of the human preaccess vein after arteriovenous fistula (AVF) creation is poorly understood. This limits our ability to design efficacious therapies to improve maturation outcomes.
Methods: Bulk RNA sequencing (RNA-seq) followed by paired bioinformatic analyses and validation assays were performed in 76 longitudinal vascular biopsies (veins and AVFs) from 38 patients with stage 5 chronic kidney disease or end-stage kidney disease undergoing surgeries for 2-stage AVF creation (19 matured, 19 failed).
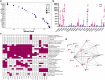
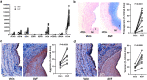
Results: A total of 3637 transcripts were differentially expressed between veins and AVFs independent of maturation outcomes, with 80% upregulated in fistulas. The postoperative transcriptome demonstrated transcriptional activation of basement membrane and interstitial extracellular matrix (ECM) components, including preexisting and novel collagens, proteoglycans, hemostasis factors, and angiogenesis regulators. A postoperative intramural cytokine storm involved >80 chemokines, interleukins, and growth factors. Postoperative changes in ECM expression were differentially distributed in the AVF wall, with proteoglycans and fibrillar collagens predominantly found in the intima and media, respectively. Interestingly, upregulated matrisome genes were enough to make a crude separation of AVFs that failed from those with successful maturation. We identified 102 differentially expressed genes (DEGs) in association with AVF maturation failure, including upregulation of network collagen VIII in medial smooth muscle cells (SMCs) and downregulation of endothelial-predominant transcripts and ECM regulators.
Conclusion: This work delineates the molecular changes that characterize venous remodeling after AVF creation and those relevant to maturation failure. We provide an essential framework to streamline translational models and our search for antistenotic therapies.
Keywords: arteriovenous fistula; extracellular matrix; maturation; transcriptomics.
Figures






Similar articles
-
Transcriptomics of Human Arteriovenous Fistula Failure: Genes Associated With Nonmaturation.Am J Kidney Dis. 2019 Jul;74(1):73-81. doi: 10.1053/j.ajkd.2018.12.035. Epub 2019 Feb 27. Am J Kidney Dis. 2019. PMID: 30826088 Free PMC article.
-
Temporal regulation of notch activation improves arteriovenous fistula maturation.J Transl Med. 2022 Nov 23;20(1):543. doi: 10.1186/s12967-022-03727-7. J Transl Med. 2022. PMID: 36419038 Free PMC article.
-
Analyses of hemodialysis arteriovenous fistula geometric configuration and its associations with maturation and reintervention.J Vasc Surg. 2021 May;73(5):1778-1786.e1. doi: 10.1016/j.jvs.2020.09.033. Epub 2020 Oct 20. J Vasc Surg. 2021. PMID: 33091518 Free PMC article.
-
Building a Scaffold for Arteriovenous Fistula Maturation: Unravelling the Role of the Extracellular Matrix.Int J Mol Sci. 2023 Jun 28;24(13):10825. doi: 10.3390/ijms241310825. Int J Mol Sci. 2023. PMID: 37446003 Free PMC article. Review.
-
Arteriovenous conduits for hemodialysis: how to better modulate the pathophysiological vascular response to optimize vascular access durability.Am J Physiol Renal Physiol. 2019 May 1;316(5):F794-F806. doi: 10.1152/ajprenal.00440.2018. Epub 2019 Feb 20. Am J Physiol Renal Physiol. 2019. PMID: 30785348 Free PMC article. Review.
Cited by
-
Hemodynamics are associated with subsequent lumen remodeling and clinical maturation of hemodialysis arteriovenous fistula.Sci Rep. 2025 Feb 19;15(1):6131. doi: 10.1038/s41598-025-89896-z. Sci Rep. 2025. PMID: 39972115 Free PMC article.
-
Single-Cell Analyses Offer Insights into the Different Remodeling Programs of Arteries and Veins.Cells. 2024 May 7;13(10):793. doi: 10.3390/cells13100793. Cells. 2024. PMID: 38786017 Free PMC article.
-
Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure.Antioxidants (Basel). 2023 Dec 19;13(1):5. doi: 10.3390/antiox13010005. Antioxidants (Basel). 2023. PMID: 38275625 Free PMC article.
-
Extracellular matrix in vascular homeostasis and disease.Nat Rev Cardiol. 2025 May;22(5):333-353. doi: 10.1038/s41569-024-01103-0. Epub 2025 Jan 2. Nat Rev Cardiol. 2025. PMID: 39743560 Review.
-
Egr‑1 promotes the proliferation and migration of vascular smooth muscle cells by transcriptionally activating Egr‑2 in arteriovenous fistulas.Int J Mol Med. 2025 Sep;56(3):127. doi: 10.3892/ijmm.2025.5568. Epub 2025 Jun 27. Int J Mol Med. 2025. PMID: 40576118 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

