Eculizumab Rescue Therapy in Patients With Recurrent Atypical Hemolytic Uremic Syndrome After Kidney Transplantation
- PMID: 37069997
- PMCID: PMC10105043
- DOI: 10.1016/j.ekir.2023.01.016
Eculizumab Rescue Therapy in Patients With Recurrent Atypical Hemolytic Uremic Syndrome After Kidney Transplantation
Abstract
Introduction: Since 2016, kidney transplantation in patients with atypical hemolytic uremic syndrome (aHUS) in the Netherlands is performed without eculizumab prophylaxis. Eculizumab is given in case of posttransplant aHUS recurrence. Eculizumab therapy is monitored in the CUREiHUS study.
Methods: All participating kidney transplant patients who received eculizumab therapy for a suspected posttransplant aHUS recurrence were evaluated. Overall recurrence rate was monitored prospectively at Radboud University Medical Center.
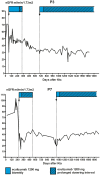
Results: In the period from January 2016 until October 2020, we included 15 (12 females, 3 males; median age 42 years, range 24-66 years) patients with suspected aHUS recurrence after kidney transplantation in this study. The time interval to recurrence showed a bimodal distribution. Seven patients presented early after transplantation (median 3 months, range 0.3-8.8 months), with typical aHUS features: rapid loss of estimated glomerular filtration rate (eGFR) and laboratory signs of thrombotic microangiopathy (TMA). Eight patients presented late (median 46 months, range 18-69 months) after transplantation. Of these, only 3 patients had systemic TMA, whereas 5 patients presented with slowly deteriorating eGFR without systemic TMA. Treatment with eculizumab resulted in improvement or stabilization of eGFR in 14 patients. Eculizumab discontinuation was tried in 7 patients; however, it was successful only in 3. At the end of the follow-up (median 29 months, range 3-54 months after start of eculizumab), 6 patients had eGFR <30 ml/min per 1.73 m2. Graft loss had occurred in 3 of them. Overall, aHUS recurrence rate without eculizumab prophylaxis was 23%.
Conclusions: Rescue treatment of posttransplant aHUS recurrence is effective; however, some patients suffer from irreversible loss of kidney function, likely caused by delayed diagnosis and treatment and/or too aggressive discontinuation of eculizumab. Physicians should be aware that recurrence of aHUS can present without evidence of systemic TMA.
Keywords: atypical hemolytic uremic syndrome; eculizumab; kidney transplantation; thrombotic microangiopathy.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

