Lycopene inhibits endothelial-to-mesenchymal transition of choroidal vascular endothelial cells in laser-induced mouse choroidal neovascularization
- PMID: 37070131
- PMCID: PMC10183704
- DOI: 10.1111/jcmm.17730
Lycopene inhibits endothelial-to-mesenchymal transition of choroidal vascular endothelial cells in laser-induced mouse choroidal neovascularization
Abstract
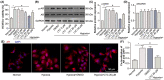
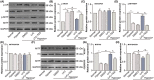
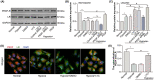
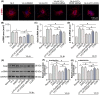
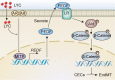
Choroidal neovascularization (CNV), is a major cause of irreversible blindness among the elderly population in developed countries, which is resulted from subretinal fibrosis without effective therapeutic strategies. Endothelial-to-mesenchymal transition (EndMT) of choroidal vascular endothelial cells (CVECs) contributes to subretinal fibrosis. Lycopene (LYC), a non-pro-vitamin A carotenoid, plays an anti-fibrotic role. Herein, we explored the effect and mechanism of LYC on the EndMT of CVECs during CNV. Firstly, LYC inhibited EndMT in hypoxic human choroidal endothelial cells (HCVECs). Meanwhile, LYC inhibited proliferation, androgen receptor (AR) expression and nuclear localization in hypoxic HCVECs. Then LYC-inhibited AR promotes the activation of microphthalmia-associated transcription factor (MITF) in hypoxic HCVECs. In addition, LYC down-regulated AR and induced MITF up-regulated pigment epithelium-derived factor (PEDF) transcription and expression in hypoxic HCVECs. Moreover, LYC-induced PEDF bound to laminin receptor (LR), inhibiting EndMT of hypoxic HCVECs via down-regulating protein kinase B (AKT)/β-catenin pathway. In vivo, LYC alleviated mouse laser-induced subretinal fibrosis secondary to CNV via up-regulating PEDF without any ocular or systemic toxicity. These results indicate that LYC inhibits EndMT of CVECs via modulating AR/MITF/PEDF/LR/AKT/β-catenin pathway, showing LYC is a promising therapeutic agent for CNV.
Keywords: choroidal neovascularization; choroidal vascular endothelial cells; endothelial to mesenchymal transition; lycopene.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All of the authors declare that there is no conflict of interests.
Figures




Similar articles
-
CBX7 promotes choroidal neovascularization by activating the HIF-1α/VEGF pathway in choroidal vascular endothelial cells.Exp Eye Res. 2024 Oct;247:110057. doi: 10.1016/j.exer.2024.110057. Epub 2024 Aug 22. Exp Eye Res. 2024. PMID: 39179168
-
CSF1/CSF1R-mediated Crosstalk Between Choroidal Vascular Endothelial Cells and Macrophages Promotes Choroidal Neovascularization.Invest Ophthalmol Vis Sci. 2021 Mar 1;62(3):37. doi: 10.1167/iovs.62.3.37. Invest Ophthalmol Vis Sci. 2021. PMID: 33764399 Free PMC article.
-
Fruquintinib inhibits VEGF/VEGFR2 axis of choroidal endothelial cells and M1-type macrophages to protect against mouse laser-induced choroidal neovascularization.Cell Death Dis. 2020 Nov 27;11(11):1016. doi: 10.1038/s41419-020-03222-1. Cell Death Dis. 2020. PMID: 33247124 Free PMC article.
-
ANGPTL4 promotes choroidal neovascularization and subretinal fibrosis through the endothelial‒mesenchymal transition.Int Ophthalmol. 2024 Nov 26;44(1):441. doi: 10.1007/s10792-024-03348-7. Int Ophthalmol. 2024. PMID: 39586852
-
Pigment Epithelium-Derived Factor as a Possible Treatment Agent for Choroidal Neovascularization.Oxid Med Cell Longev. 2020 Mar 6;2020:8941057. doi: 10.1155/2020/8941057. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32215180 Free PMC article. Review.
Cited by
-
Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model.Biomedicines. 2023 Sep 2;11(9):2445. doi: 10.3390/biomedicines11092445. Biomedicines. 2023. PMID: 37760886 Free PMC article.
-
Plasticity of Human Microglia and Brain Perivascular Macrophages in Aging and Alzheimer's Disease.medRxiv [Preprint]. 2024 Dec 5:2023.10.25.23297558. doi: 10.1101/2023.10.25.23297558. medRxiv. 2024. PMID: 39677435 Free PMC article. Preprint.
-
Clinicopathological-genetic features of neutral lipid storage disease with myopathy from a Chinese neuromuscular center.Orphanet J Rare Dis. 2025 Jul 1;20(1):322. doi: 10.1186/s13023-025-03861-7. Orphanet J Rare Dis. 2025. PMID: 40598302 Free PMC article.
-
Wnt5a/β-catenin-mediated epithelial-mesenchymal transition: a key driver of subretinal fibrosis in neovascular age-related macular degeneration.J Neuroinflammation. 2024 Mar 26;21(1):75. doi: 10.1186/s12974-024-03068-w. J Neuroinflammation. 2024. PMID: 38532410 Free PMC article.
References
-
- Zhang M, Tombran‐Tink J, Yang S, Zhang X, Li X, Barnstable CJ. PEDF is an endogenous inhibitor of VEGF‐R2 angiogenesis signaling in endothelial cells. Exp Eye Res. 2021;213:108828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

