A multidisciplinary approach to the identification of the protein-RNA connectome in double-stranded RNA virus capsids
- PMID: 37070191
- PMCID: PMC10250232
- DOI: 10.1093/nar/gkad274
A multidisciplinary approach to the identification of the protein-RNA connectome in double-stranded RNA virus capsids
Abstract
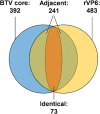
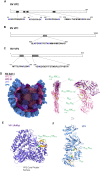
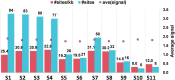
How multi-segmented double-stranded RNA (dsRNA) viruses correctly incorporate their genomes into their capsids remains unclear for many viruses, including Bluetongue virus (BTV), a Reoviridae member, with a genome of 10 segments. To address this, we used an RNA-cross-linking and peptide-fingerprinting assay (RCAP) to identify RNA binding sites of the inner capsid protein VP3, the viral polymerase VP1 and the capping enzyme VP4. Using a combination of mutagenesis, reverse genetics, recombinant proteins and in vitro assembly, we validated the importance of these regions in virus infectivity. Further, to identify which RNA segments and sequences interact with these proteins, we used viral photo-activatable ribonucleoside crosslinking (vPAR-CL) which revealed that the larger RNA segments (S1-S4) and the smallest segment (S10) have more interactions with viral proteins than the other smaller segments. Additionally, using a sequence enrichment analysis we identified an RNA motif of nine bases that is shared by the larger segments. The importance of this motif for virus replication was confirmed by mutagenesis followed by virus recovery. We further demonstrated that these approaches could be applied to a related Reoviridae member, rotavirus (RV), which has human epidemic impact, offering the possibility of novel intervention strategies for a human pathogen.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




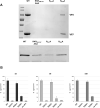


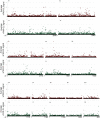
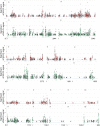
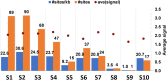

Similar articles
-
The Interaction of Bluetongue Virus VP6 and Genomic RNA Is Essential for Genome Packaging.J Virol. 2019 Feb 19;93(5):e02023-18. doi: 10.1128/JVI.02023-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541863 Free PMC article.
-
RNA Origami: Packaging a Segmented Genome in Orbivirus Assembly and Replication.Viruses. 2021 Sep 15;13(9):1841. doi: 10.3390/v13091841. Viruses. 2021. PMID: 34578422 Free PMC article. Review.
-
Atomic Structure of the Trichomonas vaginalis Double-Stranded RNA Virus 2.mBio. 2021 Mar 30;12(2):e02924-20. doi: 10.1128/mBio.02924-20. mBio. 2021. PMID: 33785622 Free PMC article.
-
Disruption of Specific RNA-RNA Interactions in a Double-Stranded RNA Virus Inhibits Genome Packaging and Virus Infectivity.PLoS Pathog. 2015 Dec 8;11(12):e1005321. doi: 10.1371/journal.ppat.1005321. eCollection 2015 Dec. PLoS Pathog. 2015. PMID: 26646790 Free PMC article.
-
Functional mapping of bluetongue virus proteins and their interactions with host proteins during virus replication.Cell Biochem Biophys. 2008;50(3):143-57. doi: 10.1007/s12013-008-9009-4. Epub 2008 Feb 26. Cell Biochem Biophys. 2008. PMID: 18299997 Review.
Cited by
-
Recruitment of multi-segment genomic RNAs by Bluetongue virus requires a preformed RNA network.Nucleic Acids Res. 2024 Aug 12;52(14):8500-8514. doi: 10.1093/nar/gkae404. Nucleic Acids Res. 2024. PMID: 38769067 Free PMC article.
-
RNA genome packaging and capsid assembly of bluetongue virus visualized in host cells.Cell. 2024 Apr 25;187(9):2236-2249.e17. doi: 10.1016/j.cell.2024.03.007. Epub 2024 Apr 12. Cell. 2024. PMID: 38614100 Free PMC article.
References
-
- Ford R.J., Barker A.M., Bakker S.E., Coutts R.H., Ranson N.A., Phillips S.E., Pearson A.R., Stockley P.G.. Sequence-specific, RNA-protein interactions overcome electrostatic barriers preventing assembly of satellite tobacco necrosis virus coat protein. J. Mol. Biol. 2013; 425:1050–1064. - PMC - PubMed
-
- Patel N., White S.J., Thompson R.F., Bingham R., Weiss E.U., Maskell D.P., Zlotnick A., Dykeman E., Tuma R., Twarock R.et al. .. HBV RNA pre-genome encodes specific motifs that mediate interactions with the viral core protein that promote nucleocapsid assembly. Nat. Microbiol. 2017; 2:17098. - PMC - PubMed
-
- Poranen M.M., Tuma R.. Self-assembly of double-stranded RNA bacteriophages. Virus. Res. 2004; 101:93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

