Nonmuscle Myosin IIA Regulates the Precise Alignment of Hexagonal Eye Lens Epithelial Cells During Fiber Cell Formation and Differentiation
- PMID: 37070941
- PMCID: PMC10123325
- DOI: 10.1167/iovs.64.4.20
Nonmuscle Myosin IIA Regulates the Precise Alignment of Hexagonal Eye Lens Epithelial Cells During Fiber Cell Formation and Differentiation
Abstract
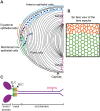
Purpose: Epithelial cells in the equatorial region of the ocular lens undergo a remarkable transition from randomly packed cells into precisely aligned and hexagon-shaped cells organized into meridional rows. We investigated the function of nonmuscle myosin IIA (encoded by Myh9) in regulating equatorial epithelial cell alignment to form meridional rows during secondary fiber cell morphogenesis.
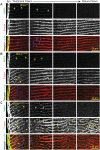
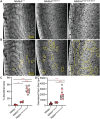
Methods: We used genetic knock-in mice to study a common human Myh9 mutation, E1841K, in the rod domain. The E1841K mutation disrupts bipolar filament assembly. Lens shape, clarity, and stiffness were evaluated, and Western blots were used to determine the level of normal and mutant myosins. Cryosections and lens whole mounts were stained and imaged by confocal microscopy to investigate cell shape and organization.
Results: We observed no obvious changes in lens size, shape, and biomechanical properties (stiffness and resilience) between the control and nonmuscle myosin IIA-E1841K mutant mice at 2 months of age. Surprisingly, we found misalignment and disorder of fiber cells in heterozygous and homozygous mutant lenses. Further analysis revealed misshapen equatorial epithelial cells that cause disorientation of the meridional rows before fiber cell differentiation in homozygous mutant lenses.
Conclusions: Our data indicate that nonmuscle myosin IIA bipolar filament assembly is required for the precise alignment of the meridional rows at the lens equator and that the organization of lens fiber cells depends on the proper patterning of meridional row epithelial cells. These data also suggest that lens fiber cell organization and a hexagonal shape are not required for normal lens size, shape transparency, or biomechanical properties.
Conflict of interest statement
Disclosure:
Figures





References
-
- Lovicu F, Robinson M. Development of the ocular lens. Cambridge, UK: Cambridge University Press; 2004.
-
- Lovicu F, Robinson M. Development of the ocular lens. Cambridge, UK: Cambridge University Press; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

